A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory
- PMID: 25378645
- PMCID: PMC4669853
- DOI: 10.1126/scitranslmed.3009185
A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory
Abstract
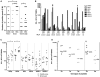
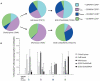
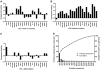
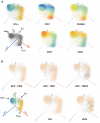
A protective vaccine against hepatitis C virus (HCV) remains an unmet clinical need. HCV infects millions of people worldwide and is a leading cause of liver cirrhosis and hepatocellular cancer. Animal challenge experiments, immunogenetics studies, and assessment of host immunity during acute infection highlight the critical role that effective T cell immunity plays in viral control. In this first-in-man study, we have induced antiviral immunity with functional characteristics analogous to those associated with viral control in natural infection, and improved upon a vaccine based on adenoviral vectors alone. We assessed a heterologous prime-boost vaccination strategy based on a replicative defective simian adenoviral vector (ChAd3) and modified vaccinia Ankara (MVA) vector encoding the NS3, NS4, NS5A, and NS5B proteins of HCV genotype 1b. Analysis used single-cell mass cytometry and human leukocyte antigen class I peptide tetramer technology in healthy human volunteers. We show that HCV-specific T cells induced by ChAd3 are optimally boosted with MVA, and generate very high levels of both CD8(+) and CD4(+) HCV-specific T cells targeting multiple HCV antigens. Sustained memory and effector T cell populations are generated, and T cell memory evolved over time with improvement of quality (proliferation and polyfunctionality) after heterologous MVA boost. We have developed an HCV vaccine strategy, with durable, broad, sustained, and balanced T cell responses, characteristic of those associated with viral control, paving the way for the first efficacy studies of a prophylactic HCV vaccine.
Trial registration: ClinicalTrials.gov NCT01296451.
Copyright © 2014, American Association for the Advancement of Science.
Figures



References
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N. Engl. J. Med. 2001;345:41–52. - PubMed
-
- Gane EJ, Agarwal K. Directly Acting Antivirals (DAAs) for the Treatment of Chronic Hepatitis C Virus Infection in Liver Transplant Patients: “A Flood of Opportunity”. Am. J. Transplant. 2014;14:994–1002. - PubMed
-
- Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N, Rossaro L, Anderson FH, Jacobson IM, Rubin R, Koury K, Pedicone LD, Brass CA, Chaudhri E, Albrecht JK. SPRINT-1 investigators, Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

