Exposure to High Doses of Lipopolysaccharide during Ovalbumin Sensitization Prevents the Development of Allergic Th2 Responses to a Dietary Antigen
- PMID: 25378805
- PMCID: PMC4217231
- DOI: 10.1293/tox.2014-0023
Exposure to High Doses of Lipopolysaccharide during Ovalbumin Sensitization Prevents the Development of Allergic Th2 Responses to a Dietary Antigen
Erratum in
-
Errata (Printer's correction).J Toxicol Pathol. 2016 Jan;29(1):74. Epub 2016 Feb 17. J Toxicol Pathol. 2016. PMID: 26989306 Free PMC article.
Abstract
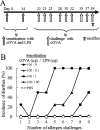
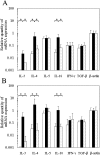
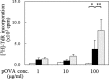
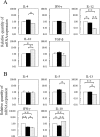
Food allergies are driven by aberrant T helper (Th) 2 cells. Lipopolysaccharide (LPS) influences the development of Th2-mediated diseases, but its role in food allergy and tolerance remains unclear. To address this issue, we established mouse models presenting allergic or tolerant responses to ovalbumin (OVA). Mice sensitized with crude OVA developed Th2 responses including acute diarrhea, increases in serum OVA-specific IgE, dominant production of serum OVA-specific IgG1, increases in Th2-type cytokines and proliferation of mast cells in duodenal and colonic tissues. Sensitization of mice with crude OVA and LPS abrogated Th2-type responses observed in allergic mice. The level of OVA-specific proliferation in mesenteric lymph node CD4(+) T cells was comparable in allergic and tolerant mice, indicating that the tolerance is not caused by anergy and apoptosis of antigen-primed T cells. Expression of Th1- and Th2-type cytokines was suppressed in whole spleen cells and/or purified spleen CD4(+) T cells of tolerant mice, indicating that the tolerance was not caused by the shift from Th2 to Th1. On the other hand, interleukin (IL)-10, a regulatory cytokine produced by regulatory T cells, was upregulated in whole spleen cells and purified spleen CD4(+) T cells of tolerant mice. Furthermore, spleen CD4(+) T cells from tolerant mice suppressed the growth of CD4(+) T cells from DO11.10 mice in co-culture. These results indicate that tolerance is induced in allergic mice by simultaneous exposure to LPS during sensitization with OVA and that a population of T cells producing IL-10 plays an important role in the tolerance induction.
Keywords: allergy; lipopolysaccharide; ovalbumin; tolerance.
Figures




References
-
- Sampson HA, and Burks AW. Mechanisms of food allergy. Annu Rev Nutr. 16: 161–177. 1996. - PubMed
-
- Sampson HA, and Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 100: 444–451. 1997. - PubMed
-
- Verdú EF, Bercík P, Bergonzelli GE, Huang XX, Blennerhasset P, Rochat F, Fiaux M, Mansourian R, Corthésy-Theulaz I, and Collins SM. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 127: 826–837. 2004. - PubMed
-
- Moon A, and Kleinman RE. Allergic gastroenteropathy in children. Ann Allergy Asthma Immunol. 74: 5–12, quiz 12–16. 1995. - PubMed
-
- Brix S, Kjaer TM, Barkholt V, and Frøkiaer H. Lipopolysaccharide contamination of beta-lactoglobulin affects the immune response against intraperitoneally and orally administered antigen. Int Arch Allergy Immunol. 135: 216–220. 2004. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
