Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress
- PMID: 25379019
- PMCID: PMC4212247
- DOI: 10.1016/j.neo.2014.08.006
Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress
Abstract
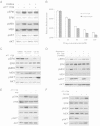
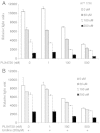
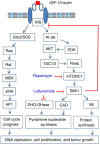
Leflunomide is a novel immunomodulatory drug prescribed for treating rheumatoid arthritis. It inhibits the activity of protein tyrosine kinases and dihydroorotate dehydrogenase, a rate-limiting enzyme in the pyrimidine nucleotide synthesis pathway. Here, we report that A77 1726, the active metabolite of leflunomide, inhibited the phosphorylation of ribosomal protein S6 and two other substrates of S6K1, insulin receptor substrate-1 and carbamoyl phosphate synthetase 2, in an A375 melanoma cell line. A77 1726 increased the phosphorylation of AKT, p70 S6 (S6K1), ERK1/2, and MEK through the feedback activation of the IGF-1 receptor-mediated signaling pathway. In vitro kinase assay revealed that leflunomide and A77 1726 inhibited S6K1 activity with IC50 values of approximately 55 and 80 μM, respectively. Exogenous uridine partially blocked A77 1726-induced inhibition of A375 cell proliferation. S6K1 knockdown led to the inhibition of A375 cell proliferation but did not potentiate the antiproliferative effect of A77 1726. A77 1726 stimulated bromodeoxyuridine incorporation in A375 cells but arrested the cell cycle in the S phase, which was reversed by addition of exogenous uridine or by MAP kinase pathway inhibitors but not by rapamycin and LY294002 (a phosphoinositide 3-kinase inhibitor). These observations suggest that A77 1726 accelerates cell cycle entry into the S phase through MAP kinase activation and that pyrimidine nucleotide depletion halts the completion of the cell cycle. Our study identified a novel molecular target of A77 1726 and showed that the inhibition of S6K1 activity was in part responsible for its antiproliferative activity. Our study also provides a novel mechanistic insight into A77 1726-induced cell cycle arrest in the S phase.
Figures




References
-
- Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010;16:4325–4330. - PubMed
-
- Emerling BM, Akcakanat A. Targeting PI3K/mTOR signaling in cancer. Cancer Res. 2011;71:7351–7359. - PubMed
-
- Xu X, Blinder L, Shen J, Gong H, Finnegan A, Williams JW, Chong AS. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J Immunol. 1997;159:167–174. - PubMed
-
- Xu X, Shen J, Mall JW, Myers JA, Huang W, Blinder L, Saclarides TJ, Williams JW, Chong AS. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem Pharmacol. 1999;58:1405–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

