Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury
- PMID: 25379095
- PMCID: PMC4189213
- DOI: 10.1063/1.4891098
Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury
Abstract
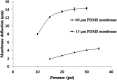
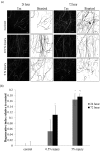
A new model for studying localised axonal stretch injury is presented, using a microfluidic device to selectively culture axons on a thin, flexible poly (dimethylsiloxane) membrane which can be deflected upward to stretch the axons. A very mild (0.5% strain) or mild stretch injury (5% strain) was applied to primary cortical neurons after 7 days growth in vitro. The extent of distal degeneration was quantified using the degenerative index (DI, the ratio of fragmented axon area to total axon area) of axons fixed at 24 h and 72 h post injury (PI), and immunolabelled for the axon specific, microtubule associated protein-tau. At 24 h PI following very mild injuries (0.5%), the majority of the axons remained intact and healthy with no significant difference in DI when compared to the control, but at 72 h PI, the DI increased significantly (DI = 0.11 ± 0.03). Remarkably, dendritic beading in the somal compartment was observed at 24 h PI, indicative of dying back degeneration. When the injury level was increased (5% stretch, mild injury), microtubule fragmentation along the injured axons was observed, with a significant increase in DI at 24 h PI (DI = 0.17 ± 0.02) and 72 h PI (DI = 0.18 ± 0.01), relative to uninjured axons. The responses observed for both mild and very mild injuries are similar to those observed in the in vivo models of traumatic brain injury, suggesting that this model can be used to study neuronal trauma and will provide new insights into the cellular and molecular alterations characterizing the neuronal response to discrete axonal injury.
Figures



Similar articles
-
Mild and repetitive very mild axonal stretch injury triggers cystoskeletal mislocalization and growth cone collapse.PLoS One. 2017 May 4;12(5):e0176997. doi: 10.1371/journal.pone.0176997. eCollection 2017. PLoS One. 2017. PMID: 28472086 Free PMC article.
-
Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy.J Neurotrauma. 2005 Oct;22(10):1081-91. doi: 10.1089/neu.2005.22.1081. J Neurotrauma. 2005. PMID: 16238485
-
Dorsal root ganglion neurons recapitulate the traumatic axonal injury of CNS neurons in response to a rapid stretch in vitro.Front Cell Neurosci. 2023 Mar 29;17:1111403. doi: 10.3389/fncel.2023.1111403. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37066078 Free PMC article.
-
White matter involvement after TBI: Clues to axon and myelin repair capacity.Exp Neurol. 2016 Jan;275 Pt 3:328-333. doi: 10.1016/j.expneurol.2015.02.011. Epub 2015 Feb 16. Exp Neurol. 2016. PMID: 25697845 Review.
-
Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments.Front Cell Neurosci. 2014 Dec 17;8:429. doi: 10.3389/fncel.2014.00429. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25565963 Free PMC article. Review.
Cited by
-
A Microchip for High-throughput Axon Growth Drug Screening.Micromachines (Basel). 2016 Jul;7(7):114. doi: 10.3390/mi7070114. Epub 2016 Jul 7. Micromachines (Basel). 2016. PMID: 27928514 Free PMC article.
-
[Recent advances in emerging three-dimensional in vitro models for sport-related traumatic brain injury].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Aug 25;38(4):797-804. doi: 10.7507/1001-5515.202012050. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 34459181 Free PMC article. Review. Chinese.
-
Amyloidogenic Processing of Amyloid Precursor Protein Drives Stretch-Induced Disruption of Axonal Transport in hiPSC-Derived Neurons.J Neurosci. 2021 Dec 8;41(49):10034-10053. doi: 10.1523/JNEUROSCI.2553-20.2021. Epub 2021 Oct 18. J Neurosci. 2021. PMID: 34663629 Free PMC article.
-
Microfluidic platforms for the study of neuronal injury in vitro.Biotechnol Bioeng. 2018 Apr;115(4):815-830. doi: 10.1002/bit.26519. Epub 2018 Feb 21. Biotechnol Bioeng. 2018. PMID: 29251352 Free PMC article. Review.
-
A fluid-walled microfluidic platform for human neuron microcircuits and directed axotomy.Lab Chip. 2024 Jun 25;24(13):3252-3264. doi: 10.1039/d4lc00107a. Lab Chip. 2024. PMID: 38841815 Free PMC article.
References
-
- Smith D. H. and Meaney D. F., Neuroscientist 6, 483 (2000).10.1177/107385840000600611 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
