Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits
- PMID: 25380328
- PMCID: PMC4407839
- DOI: 10.1002/cne.23705
Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits
Abstract
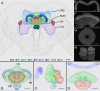
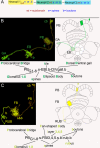
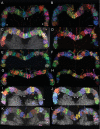
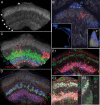
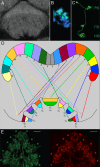
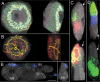
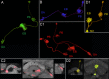
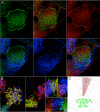
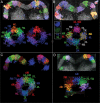
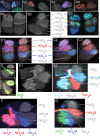
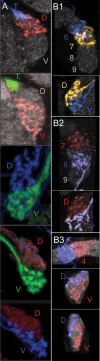
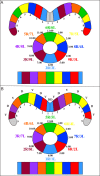
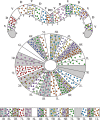
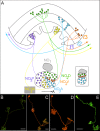
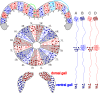
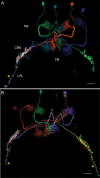
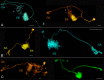
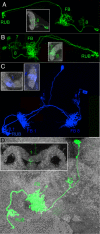
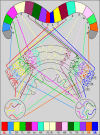
Insects exhibit an elaborate repertoire of behaviors in response to environmental stimuli. The central complex plays a key role in combining various modalities of sensory information with an insect's internal state and past experience to select appropriate responses. Progress has been made in understanding the broad spectrum of outputs from the central complex neuropils and circuits involved in numerous behaviors. Many resident neurons have also been identified. However, the specific roles of these intricate structures and the functional connections between them remain largely obscure. Significant gains rely on obtaining a comprehensive catalog of the neurons and associated GAL4 lines that arborize within these brain regions, and on mapping neuronal pathways connecting these structures. To this end, small populations of neurons in the Drosophila melanogaster central complex were stochastically labeled using the multicolor flip-out technique and a catalog was created of the neurons, their morphologies, trajectories, relative arrangements, and corresponding GAL4 lines. This report focuses on one structure of the central complex, the protocerebral bridge, and identifies just 17 morphologically distinct cell types that arborize in this structure. This work also provides new insights into the anatomical structure of the four components of the central complex and its accessory neuropils. Most strikingly, we found that the protocerebral bridge contains 18 glomeruli, not 16, as previously believed. Revised wiring diagrams that take into account this updated architectural design are presented. This updated map of the Drosophila central complex will facilitate a deeper behavioral and physiological dissection of this sophisticated set of structures.
Keywords: AB_1549585; AB_1625981; AB_528108; AB_915420; Drosophila brain; MCFO; ellipsoid body; fan-shaped body; glomerulus; nodulus.
© 2014 Wiley Periodicals, Inc.
Figures



References
-
- Baker DA, Beckingham KM, Armstrong JD. Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster. J Comp Neurol. 2007;501:756–764. - PubMed
-
- Bausenwein B, Muller NR, Heisenberg M. Behavior-dependent activity labeling in the central complex of Drosophila during controlled visual stimulation. J Comp Neurol. 1994;340:255–268. - PubMed
-
- Bausenwein B, Wolf R, Heisenberg M. Genetic dissection of optomotor behavior in Drosophila melanogaster. Studies on wild-type and the mutant optomotor-blindH31. J Neurogenet. 1986;3:87–109. - PubMed
-
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20:921–926. - PubMed
-
- Butcher NJ, Friedrich AB, Lu Z, Tanimoto H, Meinertzhagen IA. Different classes of input and output neurons reveal new features in microglomeruli of the adult Drosophila mushroom body calyx. J Comp Neurol. 2012;520:2185–2201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

