Functional Histology of Salivary Gland Pleomorphic Adenoma: An Appraisal
- PMID: 25380577
- PMCID: PMC4542802
- DOI: 10.1007/s12105-014-0581-1
Functional Histology of Salivary Gland Pleomorphic Adenoma: An Appraisal
Abstract
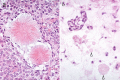
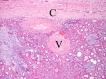
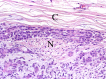
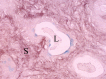
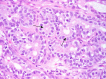
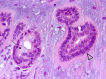
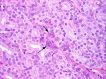
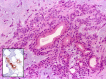
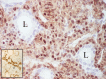
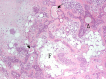
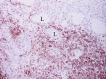
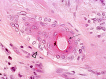
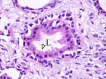
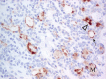
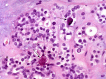
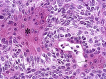
The complex microstructure of salivary gland pleomorphic adenoma is examined in relation to function. Events related to secretion of macromolecules and absorption, responses to the altered microenvironment and controversies concerning epithelial-mesenchymal transition versus modified myoepithelial differentiation are explored. Their effects on tumor cell phenotypes and arrangements are emphasized. Heterotopic differentiation and attempts at organogenesis are also considered. The approach allows interpreting microstructure independently of histogenetic perceptions, envisaging the tumor cells as a continuum, endorsing luminal structures as the principal components, and defining pleomorphic adenoma as a benign epithelial tumour characterized by variable epithelial-mesenchymal transition, secretion/differentiation and metaplasia.
Figures










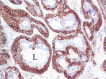
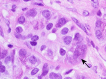
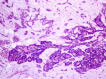

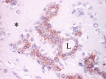
References
-
- Hand AR. Functional ultrastructure of the salivary glands. In: Sreebny LM, editor. The salivary system. Boca Raton: CRC Press; 1987. pp. 43–67.
-
- Masson P. Human tumors. Histology, diagnosis and technique. 2. Detroit: Wayne State University Press; 1970.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

