Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit
- PMID: 25380748
- PMCID: PMC4247211
- DOI: 10.1186/1471-2458-14-1159
Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit
Abstract
Background: Electronic cigarettes (e-Cigs) are an attractive long-term alternative nicotine source to conventional cigarettes. Although they may assist smokers to remain abstinent during their quit attempt, studies using first generation e-Cigs report low success rates. Second generation devices (personal vaporisers - PVs) may result in much higher quit rates, but their efficacy and safety in smoking cessation and/or reduction in clinical trials is unreported.
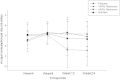
Method: We conducted a prospective proof-of-concept study monitoring modifications in smoking behaviour of 50 smokers (unwilling to quit) switched onto PVs. Participants attended five study visits: baseline, week-4, week-8, week-12 and week-24. Number of cigarettes/day (cigs/day) and exhaled carbon monoxide (eCO) levels were noted at each visit. Smoking reduction/abstinence rates, product usage, adverse events and subjective opinions of these products were also reviewed.
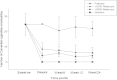
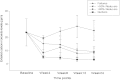
Results: Sustained 50% and 80% reduction in cigs/day at week-24 was reported in 15/50 (30%) and 7/50 (14%) participants with a reduction from 25cigs/day to 6cigs/day (p < 0.001) and 3cigs/day (p < 0.001), respectively. Smoking abstinence (self-reported abstinence from cigarette smoking verified by an eCO ≤10 ppm) at week-24 was observed in 18/50 (36%) participants, with 15/18 (83.3%) still using their PVs at the end of the study. Combined 50% reduction and smoking abstinence was shown in 33/50 (66%) participants. Throat/mouth irritation (35.6%), dry throat/mouth (28.9%), headache (26.7%) and dry cough (22.2%) were frequently reported early in the study, but waned substantially by week-24. Participants' perception and acceptance of the products was very good.
Conclusion: The use of second generation PVs substantially decreased cigarette consumption without causing significant adverse effects in smokers not intending to quit.
Trial registration: (ClinicalTrials.gov Identifier: NCT02124200).
Figures
References
-
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100(4):550–559. - PubMed
-
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. - PubMed
-
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/14/1159/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

