Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study
- PMID: 25381295
- PMCID: PMC4277676
- DOI: 10.1212/WNL.0000000000001069
Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study
Erratum in
-
Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study.Neurology. 2015 May 5;84(18):1911. doi: 10.1212/WNL.0000000000001631. Neurology. 2015. PMID: 25941203 Free PMC article. No abstract available.
Abstract
Objectives: To determine time to vigabatrin (VGB, Sabril; Lundbeck, Deerfield, IL) induced retinal damage in children with infantile spasms (IS) and to identify risk factors for VGB-induced retinal damage (VGB-RD).
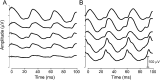
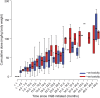
Methods: Observational cohort study including 146 participants (68 female, 81 male) with IS, an age-specific epilepsy syndrome of early infancy, treated with VGB. Participants ranged from 3 to 34.9 months of age (median 7.6 months). The median duration of VGB treatment was 16 months (range 4.6-78.5 months). Electroretinograms (ERGs) were performed according to the Standards of the International Society for Clinical Electrophysiology of Vision. Inclusion required baseline (pre-VGB or within 4 weeks of starting VGB treatment) and at least 2 follow-up ERGs. Significant reduction from baseline of the 30-Hz ERG flicker amplitude on 2 consecutive visits identified VGB-RD. Kaplan-Meier survival analyses depicted the effect of duration of VGB on VGB-RD.
Results: These data represent the largest survival analysis of children treated with VGB who did not succumb to retinal toxicity during the study. Thirty of the 146 participants (21%) showed VGB-RD. The ERG amplitude reduced with duration of VGB treatment (p = 0.0004) with no recovery after VGB cessation. With 6 and 12 months of VGB treatment, 5.3% and 13.3%, respectively, developed VGB-RD. There was neither effect of age of initiation of VGB treatment nor sex of the child on survival statistics and no significant effect of cumulative dosage on the occurrence of VGB-RD.
Conclusions: Minimizing VGB treatment to 6 months will reduce the prevalence of VGB-RD in patients with IS.
© 2014 American Academy of Neurology.
Figures


Comment in
-
Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study.Neurology. 2015 Aug 18;85(7):655-6. doi: 10.1212/01.wnl.0000471111.65017.4f. Neurology. 2015. PMID: 26283761 No abstract available.
-
Author Response.Neurology. 2015 Aug 18;85(7):656. Neurology. 2015. PMID: 26505052 No abstract available.
References
-
- Maguire MJ, Hemming K, Wild JM, Hutton JL, Marson AG. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review Epilepsia 2010;51:2423–2431. - PubMed
-
- Lundbeck. Permanent vision loss. Available at: http://sabril.net/hcp/vision_loss_data/. Accessed May 23, 2014.
-
- Agrawal S, Mayer DL, Hansen RM, Fulton AB. Visual fields in young children treated with vigabatrin. Optom Vis Sci 2009;86:767–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
