Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer
- PMID: 25381338
- PMCID: PMC5516625
- DOI: 10.1158/1078-0432.CCR-14-1913
Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer
Abstract
Background: Endocrine therapy (ET) fails to induce a response in one half of patients with hormone receptor (HR)-positive metastatic breast cancer (MBC), and almost all will eventually become refractory to ET. Circulating tumor cells (CTC) are associated with worse prognosis in patients with MBC, but enumeration alone is insufficient to predict the absolute odds of benefit from any therapy, including ET. We developed a multiparameter CTC-Endocrine Therapy Index (CTC-ETI), which we hypothesize may predict resistance to ET in patients with HR-positive MBC.
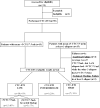
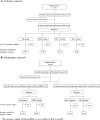
Methods: The CTC-ETI combines enumeration and CTC expression of four markers: estrogen receptor (ER), B-cell lymphoma 2 (BCL-2), Human Epidermal Growth Factor Receptor 2 (HER2), and Ki67. The CellSearch System and reagents were used to capture CTC and measure protein expression by immunofluorescent staining on CTC.
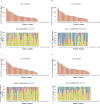
Results: The feasibility of determining CTC-ETI was initially established in vitro and then in a prospective single-institution pilot study in patients with MBC. CTC-ETI was successfully determined in 44 of 50 (88%) patients. Eighteen (41%), 9 (20%), and 17 (39%) patients had low, intermediate, and high CTC-ETI scores, respectively. Interobserver concordance of CTC-ETI determination was from 94% to 95% (Kappa statistic, 0.90-0.91). Inter- and cell-to-cell intrapatient heterogeneity of expression of each of the CTC markers was observed. CTC biomarker expression was discordant from both primary and metastatic tissues.
Conclusions: CTC expression of ER, BCL-2, HER2, and Ki67 can be reproducibly measured with high analytical validity using the CellSearch System. The clinical implications of CTC-ETI, and of the heterogeneity of CTC biomarker expression, are being evaluated in an ongoing prospective trial.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures

Comment in
-
Can circulating tumor cells predict resistance in metastatic breast cancer?Clin Cancer Res. 2015 Jun 1;21(11):2421-3. doi: 10.1158/1078-0432.CCR-14-2967. Epub 2015 Feb 2. Clin Cancer Res. 2015. PMID: 25645864 Free PMC article.
References
-
- Johnston SRD, Schiavon G. Treatment of Metastatic Breast Cancer: Endocrine. In: Harris JR, Lippman M, Morrow M, Osborne CK, editors. Diseases of the Breast. Fifth. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Tokyo: Wolters Kluwer; 2014. pp. 905–28.
-
- Goldie JH, Coldman AJ. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984;44:3643–53. - PubMed
-
- Schnipper L. Clinical implications of tumor-cell heterogeneity. N Engl J Med. 1986;314:1423–31. - PubMed
-
- Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

