Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake
- PMID: 25381433
- PMCID: PMC4223986
- DOI: 10.1093/jxb/eru232
Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake
Abstract
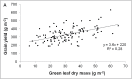
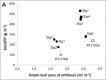
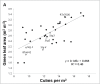
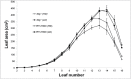
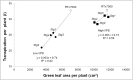
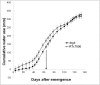
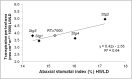
Stay-green sorghum plants exhibit greener leaves and stems during the grain-filling period under water-limited conditions compared with their senescent counterparts, resulting in increased grain yield, grain mass, and lodging resistance. Stay-green has been mapped to a number of key chromosomal regions, including Stg1, Stg2, Stg3, and Stg4, but the functions of these individual quantitative trait loci (QTLs) remain unclear. The objective of this study was to show how positive effects of Stg QTLs on grain yield under drought can be explained as emergent consequences of their effects on temporal and spatial water-use patterns that result from changes in leaf-area dynamics. A set of four Stg near-isogenic lines (NILs) and their recurrent parent were grown in a range of field and semicontrolled experiments in southeast Queensland, Australia. These studies showed that the four Stg QTLs regulate canopy size by: (1) reducing tillering via increased size of lower leaves, (2) constraining the size of the upper leaves; and (3) in some cases, decreasing the number of leaves per culm. In addition, they variously affect leaf anatomy and root growth. The multiple pathways by which Stg QTLs modulate canopy development can result in considerable developmental plasticity. The reduction in canopy size associated with Stg QTLs reduced pre-flowering water demand, thereby increasing water availability during grain filling and, ultimately, grain yield. The generic physiological mechanisms underlying the stay-green trait suggest that similar Stg QTLs could enhance post-anthesis drought adaptation in other major cereals such as maize, wheat, and rice.
Keywords: Canopy development; crop water use; drought adaptation; leaf anatomy; root architecture; sorghum; stay-green..
© The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures










References
-
- Abdulai AL, Parzies H, Kouressy M, Vaksmann M, Asch F, Brueck H. 2012. Yield stability of photoperiod sensitive sorghum (Sorghum bicolor L. Moench) accessions under diverse climatic environments. International Journal of Agricultural Research 7, 17–32.
-
- Alam MM, Hammer GL, van Oosterom EJ, Cruickshank AW, Hunt CH, Jordan DR. 2014. A physiological framework to explain genetic and environmental regulation of tillering in sorghum. New Phytologist 10.1111/nph.12767 - PubMed
-
- Bandaru V, Stewart BA, Baumhardt RL, Ambati S, Robinson CA, Schlegel A. 2006. Growing dryland grain sorghum in clumps to reduce vegetative growth and increase yield. Agronomy Journal 98, 1109–1120.
-
- Boffa JM, Taonda SJB, Dickey JB. 2000. Field-scale influence of karite (Vitellaria paradoxa) on sorghum production in the Sudan zone of Burkina Faso. Agroforestry Systems 49, 153–175.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

