Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm
- PMID: 25381571
- PMCID: PMC4426086
- DOI: 10.1007/s00441-014-2031-5
Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm
Abstract
The mammalian inner ear develops from a placodal thickening into a complex labyrinth of ducts with five sensory organs specialized to detect position and movement in space. The mammalian ear also develops a spiraled cochlear duct containing the auditory organ, the organ of Corti (OC), specialized to translate sound into hearing. Development of the OC from a uniform sheet of ectoderm requires unparalleled precision in the topological developmental engineering of four different general cell types, namely sensory neurons, hair cells, supporting cells, and general otic epithelium, into a mosaic of ten distinctly recognizable cell types in and around the OC, each with a unique distribution. Moreover, the OC receives unique innervation by ear-derived spiral ganglion afferents and brainstem-derived motor neurons as efferents and requires neural-crest-derived Schwann cells to form myelin and neural-crest-derived cells to induce the stria vascularis. This transformation of a sheet of cells into a complicated interdigitating set of cells necessitates the orchestrated expression of multiple transcription factors that enable the cellular transformation from ectoderm into neurosensory cells forming the spiral ganglion neurons (SGNs), while simultaneously transforming the flat epithelium into a tube, the cochlear duct, housing the OC. In addition to the cellular and conformational changes forming the cochlear duct with the OC, changes in the surrounding periotic mesenchyme form passageways for sound to stimulate the OC. We review molecular developmental data, generated predominantly in mice, in order to integrate the well-described expression changes of transcription factors and their actions, as revealed in mutants, in the formation of SGNs and OC in the correct position and orientation with suitable innervation. Understanding the molecular basis of these developmental changes leading to the formation of the mammalian OC and highlighting the gaps in our knowledge might guide in vivo attempts to regenerate this most complicated cellular mosaic of the mammalian body for the reconstitution of hearing in a rapidly growing population of aging people suffering from hearing loss.
Figures
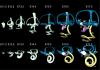

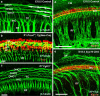
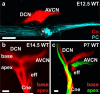
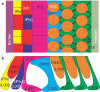
References
-
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. The Journal of comparative neurology. 2007;503:832–852. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

