Adoptive transfer of bone marrow-derived dendritic cells decreases inhibitory and regulatory T-cell differentiation and improves survival in murine polymicrobial sepsis
- PMID: 25382110
- PMCID: PMC4405323
- DOI: 10.1111/imm.12423
Adoptive transfer of bone marrow-derived dendritic cells decreases inhibitory and regulatory T-cell differentiation and improves survival in murine polymicrobial sepsis
Abstract
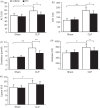
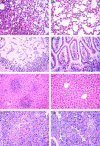
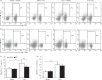
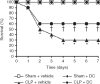
A decrease in the number of dendritic cells (DCs) is a major cause of post-sepsis immunosuppression and opportunistic infection and is closely associated with poor prognosis. Increasing the number of DCs to replenish their numbers post sepsis can improve the condition. This therapeutic approach could improve recovery after sepsis. Eighty C57BL/6 mice were subjected to sham or caecal ligation and puncture (CLP) surgery. Mice were divided into four groups: (i) Sham + vehicle, (ii) Sham + DC, (iii) CLP + vehicle, and (iv) CLP + DC. Bone-marrow-derived DCs (BMDCs) were administered at 6, 12 and 24 hr after surgery. After 3 days, we assessed serum indices of organ function (alanine aminotransferase, aspartate aminotransferase, creatinine, amylase and lipase), organ tissue histopathology (haematoxylin and eosin staining), cytokine [interferon-γ (IFN-γ), tumour necrosis factor-α, interleukin-12p70 (IL-12p70), IL-6 and IL-10] levels in the serum, programmed death-1 (PD-1) expression on T cells, regulatory T-cell differentiation in the spleen, and the survival rate (monitored for 7 days). BMDC transfer resulted in the following changes: a significant reduction in damage to the liver, kidney and pancreas in the CLP-septic mice as well as in the pathological changes seen in the liver, lung, small intestine and pancreas; significantly elevated levels of the T helper type 1 (Th1) cytokines IFN-γ and IL-12p70 in the serum; decreased levels of the Th2 cytokines IL-6 and IL-10 in the serum; reduced expression of PD-1 molecules on CD4(+) T cells; reduced the proliferation and differentiation of splenic suppressor T cells and CD4(+) CD25(+) Foxp3(+) regulatory T cells, and a significant increase in the survival rate of the septic animals. These results show that administration of BMDCs may have modulated the differentiation and immune function of T cells and contributed to alleviate immunosuppression, hence reducing organ damage and mortality post sepsis. Hence, the immunoregulatory effect of BMDC treatment has potential for the treatment of sepsis.
Keywords: dendritic cells; programmed death-1; regulatory T cells; sepsis.
© 2014 John Wiley & Sons Ltd.
Figures

Similar articles
-
Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis.J Immunol. 2011 Jan 15;186(2):977-86. doi: 10.4049/jimmunol.1001147. Epub 2010 Dec 15. J Immunol. 2011. PMID: 21160046
-
Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy.J Leukoc Biol. 2005 Sep;78(3):656-64. doi: 10.1189/jlb.1104631. Epub 2005 Jun 16. J Leukoc Biol. 2005. PMID: 15961574
-
BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo.Int Arch Allergy Immunol. 2009;150(3):210-20. doi: 10.1159/000222673. Epub 2009 Jun 3. Int Arch Allergy Immunol. 2009. PMID: 19494518
-
Alloantigen specific T regulatory cells in transplant tolerance.Int Immunopharmacol. 2009 May;9(5):570-4. doi: 10.1016/j.intimp.2009.01.016. Epub 2009 Jan 29. Int Immunopharmacol. 2009. PMID: 19539571 Review.
-
Stability of Regulatory T Cells Undermined or Endorsed by Different Type-1 Cytokines.Adv Exp Med Biol. 2015;850:17-30. doi: 10.1007/978-3-319-15774-0_2. Adv Exp Med Biol. 2015. PMID: 26324343 Review.
Cited by
-
PINK1 protects against dendritic cell dysfunction during sepsis through the regulation of mitochondrial quality control.Mol Med. 2023 Feb 21;29(1):25. doi: 10.1186/s10020-023-00618-5. Mol Med. 2023. PMID: 36809929 Free PMC article.
-
CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State.Front Immunol. 2020 Jul 7;11:1364. doi: 10.3389/fimmu.2020.01364. eCollection 2020. Front Immunol. 2020. PMID: 32733454 Free PMC article. Review.
-
Publication Trends of Research on Sepsis and Host Immune Response during 1999-2019: A 20-year Bibliometric Analysis.Int J Biol Sci. 2020 Jan 1;16(1):27-37. doi: 10.7150/ijbs.37496. eCollection 2020. Int J Biol Sci. 2020. PMID: 31892843 Free PMC article. Review.
-
Crosstalk between Dendritic Cells and Immune Modulatory Agents against Sepsis.Genes (Basel). 2020 Mar 18;11(3):323. doi: 10.3390/genes11030323. Genes (Basel). 2020. PMID: 32197507 Free PMC article. Review.
-
Migration inhibitory factor and cluster of differentiation 74-mediated dendritic cell apoptosis exacerbates acute acetaminophen-induced liver injury.Immun Inflamm Dis. 2023 Apr;11(4):e840. doi: 10.1002/iid3.840. Immun Inflamm Dis. 2023. PMID: 37102665 Free PMC article.
References
-
- Perl M, Chung CS, Garber M, Huang X, Ayala A. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front Biosci. 2006;11:272–99. - PubMed
-
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. - PubMed
-
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

