Machine Learning in Medical Imaging
- PMID: 25382956
- PMCID: PMC4220564
- DOI: 10.1109/MSP.2010.936730
Machine Learning in Medical Imaging
Figures







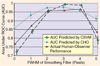
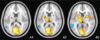



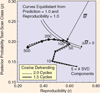
References
-
- Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning. New York: Springer-Verlag; 2003.
-
- Scholkopf B, Smola AJ. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. Cambridge, MA: MIT Press; 2001. p. 626.
-
- Wernick MN. “Pattern classification by convex analysis”. J. Opt. Soc. Amer. A, Opt. Image Sci. 1991;8:1874–1880.
-
- Vapnik VN. Statistical Learning Theory. New York: Wiley; 1998.
-
- Tipping ME. “Sparse Bayesian learning and the relevance vector machine”. J. Mach. Learn. Res. 2001 Sep;1:211–244.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
