Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells
- PMID: 25383044
- PMCID: PMC4224689
- DOI: 10.1186/s12935-014-0096-6
Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells
Abstract
Background: Ursolic acid (UA), a plant extract used in traditional Chinese medicine, exhibits potential anticancer effects in various human cancer cell lines in vitro. In the present study, we evaluated the anti-tumoral properties of UA against gallbladder carcinoma and investigated the potential mechanisms responsible for its effects on proliferation, cell cycle arrest and apoptosis in vitro.
Methods: The anti-tumor activity of UA against GBC-SD and SGC-996 cells was assessed using MTT and colony formation assays. An annexin V/PI double-staining assay was used to detect cell apoptosis. Cell cycle changes were detected using flow cytometry. Rhodamine 123 staining was used to assess the mitochondrial membrane potential (ΔΨm) and validate UA's ability to induce apoptosis in both cell lines. The effectiveness of UA in gallbladder cancer was further verified in vivo by establishing a xenograft GBC model in nude mice. Finally, the expression levels of cell cycle- and apoptosis-related proteins were analyzed by western blotting.
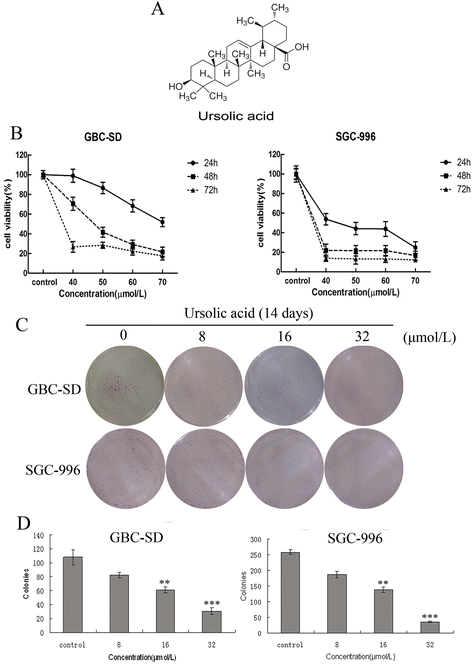
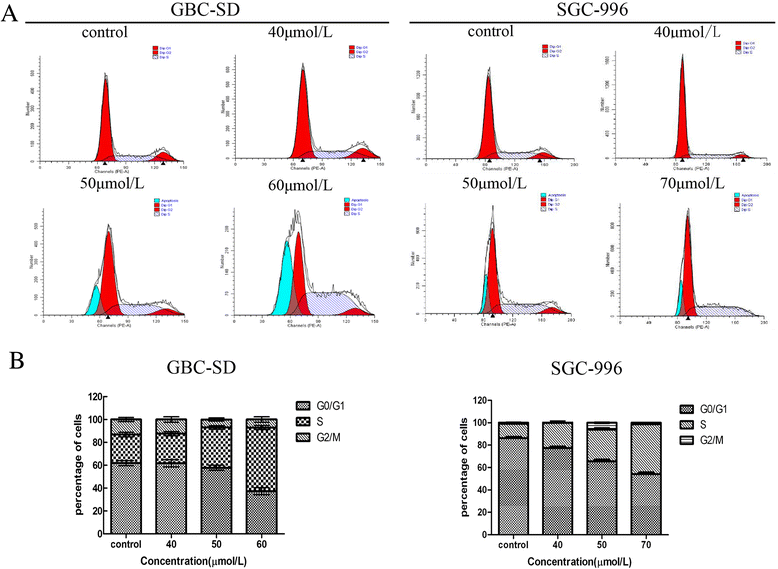
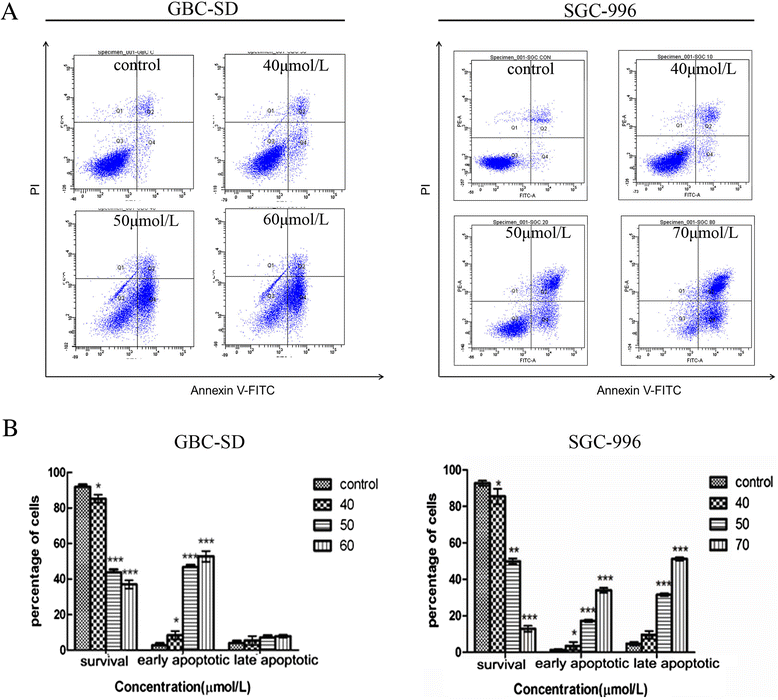
Results: Our results suggest that UA can significantly inhibit the growth of gallbladder cancer cells. MTT and colony formation assays indicated dose-dependent decreases in cell proliferation. S-phase arrest was observed in both cell lines after treatment with UA. Annexin V/PI staining suggested that UA induced both early and late phases of apoptosis. UA also decreased ΔΨm and altered the expression of molecules regulating the cell cycle and apoptosis. In vivo study showed intraperitoneally injection of UA can significantly inhibited the growth of xenograft tumor in nude mice and the inhibition efficiency is dose related. Activation of caspase-3,-9 and PARP indicated that mitochondrial pathways may be involved in UA-induced apoptosis.
Conclusions: Taken together, these results suggest that UA exhibits significant anti-tumor effects by suppressing cell proliferation, promoting apoptosis and inducing 7cell cycle arrest both in vitro and in vivo. It may be a potential agent for treating gallbladder cancer.
Keywords: Apoptosis; Cell cycle; Gallbladder cancer; Mitochondrial-mediated pathway; Proliferation; Ursolic acid.
Figures



References
-
- Rifatbegovic Z, Mesic D, Ljuca F, Zildzic M, Morankic M. Incidence and surgical treatment of cancer in gallbladder. Med Arh. 2007;61(1):30–33. - PubMed
-
- Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen W, Tian Y, Fu L, Shi D, Cheng J, Huang W, Deng W. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS ONE. 2013;8(5):e63872. doi: 10.1371/journal.pone.0063872. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

