PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments
- PMID: 25383306
- PMCID: PMC4222445
- DOI: 10.3762/bjnano.5.205
PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments
Abstract
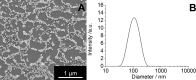
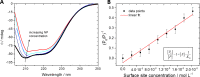
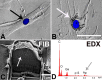
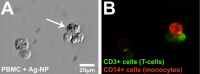
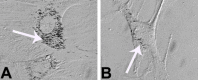
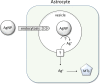
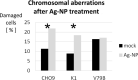
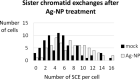
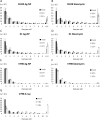
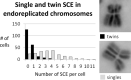
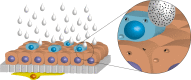
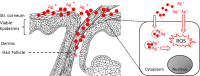
PVP-capped silver nanoparticles with a diameter of the metallic core of 70 nm, a hydrodynamic diameter of 120 nm and a zeta potential of -20 mV were prepared and investigated with regard to their biological activity. This review summarizes the physicochemical properties (dissolution, protein adsorption, dispersability) of these nanoparticles and the cellular consequences of the exposure of a broad range of biological test systems to this defined type of silver nanoparticles. Silver nanoparticles dissolve in water in the presence of oxygen. In addition, in biological media (i.e., in the presence of proteins) the surface of silver nanoparticles is rapidly coated by a protein corona that influences their physicochemical and biological properties including cellular uptake. Silver nanoparticles are taken up by cell-type specific endocytosis pathways as demonstrated for hMSC, primary T-cells, primary monocytes, and astrocytes. A visualization of particles inside cells is possible by X-ray microscopy, fluorescence microscopy, and combined FIB/SEM analysis. By staining organelles, their localization inside the cell can be additionally determined. While primary brain astrocytes are shown to be fairly tolerant toward silver nanoparticles, silver nanoparticles induce the formation of DNA double-strand-breaks (DSB) and lead to chromosomal aberrations and sister-chromatid exchanges in Chinese hamster fibroblast cell lines (CHO9, K1, V79B). An exposure of rats to silver nanoparticles in vivo induced a moderate pulmonary toxicity, however, only at rather high concentrations. The same was found in precision-cut lung slices of rats in which silver nanoparticles remained mainly at the tissue surface. In a human 3D triple-cell culture model consisting of three cell types (alveolar epithelial cells, macrophages, and dendritic cells), adverse effects were also only found at high silver concentrations. The silver ions that are released from silver nanoparticles may be harmful to skin with disrupted barrier (e.g., wounds) and induce oxidative stress in skin cells (HaCaT). In conclusion, the data obtained on the effects of this well-defined type of silver nanoparticles on various biological systems clearly demonstrate that cell-type specific properties as well as experimental conditions determine the biocompatibility of and the cellular responses to an exposure with silver nanoparticles.
Keywords: aerosols; biological properties; cell biology; nanoparticles; nanotoxicology; silver.
Figures





References
-
- Simoncic B, Tomsic B. Text Res J. 2010;80:1721–1737. doi: 10.1177/0040517510363193. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
