Microwave irradiation of nanohydroxyapatite from chicken eggshells and duck eggshells
- PMID: 25383364
- PMCID: PMC4212654
- DOI: 10.1155/2014/275984
Microwave irradiation of nanohydroxyapatite from chicken eggshells and duck eggshells
Abstract
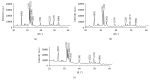
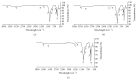
Due to similarity in composition to the mineral component of bones and human hard tissues, hydroxyapatite with chemical formula Ca10(PO4)6(OH)2 has been widely used in medical field. Both chicken and duck eggshells are mainly composed of calcium carbonate. An attempt has been made to fabricate nanohydroxyapatite (nHA) by chicken (CES) and duck eggshells (DES) as calcium carbonate source (CaCO3). CES and DES were reacted with diammonium hydrogen [(NH4)2HPO4] solution and subjected to microwave heating at 15 mins. Under the effect of microwave irradiation, nHA was produced directly in the solution and involved in crystallographic transformation. Sample characterization was done using by X-ray diffraction (XRD), fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM).
Figures

References
-
- Nejati E., Mirzadeh H., Zandi M. Synthesis and characterization of nano-hydroxyapatite rods/poly(l-lactide acid) composite scaffolds for bone tissue engineering. Composites Part A: Applied Science and Manufacturing. 2008;39(10):1589–1596. doi: 10.1016/j.compositesa.2008.05.018. - DOI
-
- Wang P., Li C., Gong H., Jiang X., Wang H., Li K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technology. 2010;203(2):315–321. doi: 10.1016/j.powtec.2010.05.023. - DOI
-
- Pramanik S., Agarwal A. K., Rai K. N. Development of high strength hydroxyapatite for hard tissue replacement. Trends in Biomaterials and Artificial Organs. 2005;19(1):46–51.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

