The participation of cortical amygdala in innate, odour-driven behaviour
- PMID: 25383519
- PMCID: PMC4231015
- DOI: 10.1038/nature13897
The participation of cortical amygdala in innate, odour-driven behaviour
Abstract
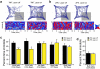
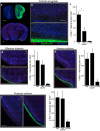
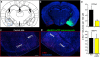
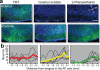
Innate behaviours are observed in naive animals without prior learning or experience, suggesting that the neural circuits that mediate these behaviours are genetically determined and stereotyped. The neural circuits that convey olfactory information from the sense organ to the cortical and subcortical olfactory centres have been anatomically defined, but the specific pathways responsible for innate responses to volatile odours have not been identified. Here we devise genetic strategies that demonstrate that a stereotyped neural circuit that transmits information from the olfactory bulb to cortical amygdala is necessary for innate aversive and appetitive behaviours. Moreover, we use the promoter of the activity-dependent gene arc to express the photosensitive ion channel, channelrhodopsin, in neurons of the cortical amygdala activated by odours that elicit innate behaviours. Optical activation of these neurons leads to appropriate behaviours that recapitulate the responses to innate odours. These data indicate that the cortical amygdala plays a critical role in generating innate odour-driven behaviours but do not preclude its participation in learned olfactory behaviours.
Figures









References
-
- Ghosh S, et al. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi:nature09945 [pii] 10.1038/nature09945. - PubMed
-
- Chamero P, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi:10.1038/nature05997. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

