A category-free neural population supports evolving demands during decision-making
- PMID: 25383902
- PMCID: PMC4294797
- DOI: 10.1038/nn.3865
A category-free neural population supports evolving demands during decision-making
Abstract
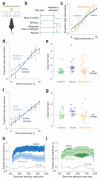
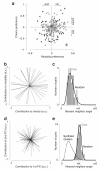
The posterior parietal cortex (PPC) receives diverse inputs and is involved in a dizzying array of behaviors. These many behaviors could rely on distinct categories of neurons specialized to represent particular variables or could rely on a single population of PPC neurons that is leveraged in different ways. To distinguish these possibilities, we evaluated rat PPC neurons recorded during multisensory decisions. Newly designed tests revealed that task parameters and temporal response features were distributed randomly across neurons, without evidence of categories. This suggests that PPC neurons constitute a dynamic network that is decoded according to the animal's present needs. To test for an additional signature of a dynamic network, we compared moments when behavioral demands differed: decision and movement. Our new state-space analysis revealed that the network explored different dimensions during decision and movement. These observations suggest that a single network of neurons can support the evolving behavioral demands of decision-making.
Figures



References
-
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. - PubMed
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Visual neuroscience. 1996;13:87–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

