N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis
- PMID: 25384424
- PMCID: PMC4763939
- DOI: 10.1096/fj.14-255208
N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis
Abstract
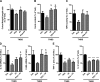
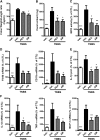
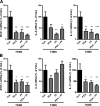
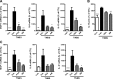
N-Palmitoylethanolamine or palmitoylethanolamide (PEA) is an anti-inflammatory compound that was recently shown to exert peroxisome proliferator-activated receptor-α-dependent beneficial effects on colon inflammation. The actions of PEA are terminated following hydrolysis by 2 enzymes: fatty acid amide hydrolase (FAAH), and the less-studied N-acylethanolamine-hydrolyzing acid amidase (NAAA). This study aims to investigate the effects of inhibiting the enzymes responsible for PEA hydrolysis in colon inflammation in order to propose a potential therapeutic target for inflammatory bowel diseases (IBDs). Two murine models of IBD were used to assess the effects of NAAA inhibition, FAAH inhibition, and PEA on macroscopic signs of colon inflammation, macrophage/neutrophil infiltration, and the expression of proinflammatory mediators in the colon, as well as on the colitis-related systemic inflammation. NAAA inhibition increases PEA levels in the colon and reduces colon inflammation and systemic inflammation, similarly to PEA. FAAH inhibition, however, does not increase PEA levels in the colon and does not affect the macroscopic signs of colon inflammation or immune cell infiltration. This is the first report of an anti-inflammatory effect of a systemically administered NAAA inhibitor. Because NAAA is the enzyme responsible for the control of PEA levels in the colon, we put forth this enzyme as a potential therapeutic target in chronic inflammation in general and IBD in particular.
Keywords: 2-AG; Crohn’s disease; N-acyltaurine; PF-3845; endocannabinoid.
© FASEB.
Figures



References
-
- Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., Mitton S., Orchard T., Rutter M., Younge L., Lees C., Ho G. T., Satsangi J., Bloom S.; IBD Section of the British Society of Gastroenterology (2011) Guidelines for the management of inflammatory bowel disease in adults. Gut 60, 571–607 - PubMed
-
- Melmed G. Y., Targan S. R. (2010) Future biologic targets for IBD: potentials and pitfalls. Nat. Rev. Gastroenterol. Hepatol. 7, 110–117 - PubMed
-
- Alhouayek M., Muccioli G. G. (2012) The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol. Med. 18, 615–625 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

