Managing the jaundiced newborn: a persistent challenge
- PMID: 25384650
- PMCID: PMC4361106
- DOI: 10.1503/cmaj.122117
Managing the jaundiced newborn: a persistent challenge
Figures

Provide lactation evaluation and support for all breastfeeding mothers.
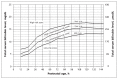
Recommendation for timing of repeat total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) measurement depends on infant’s age at measurement and how far the level is above the 95th percentile (Figure 1); higher and earlier initial levels require an earlier repeat measurement.
Perform standard clinical evaluation at all follow-up visits.
For evaluation of jaundice, see the 2004 American Academy of Pediatrics guideline.

References
-
- Sgro M, Campbell DM, Kandasamy S, et al. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics 2012;130:e886–90. - PubMed
-
- Rennie J, Burman-Roy S, Murphy MS. Neonatal jaundice: summary of NICE guidance. BMJ 2010;340:C2409. - PubMed
-
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Clinical practice guideline: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316. - PubMed
-
- Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > 35 weeks’ gestation: an update with clarifications. Pediatrics 2009;124:1193–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
