The effects of dexamethasone on post-asphyxial cerebral oxygenation in the preterm fetal sheep
- PMID: 25384775
- PMCID: PMC4270508
- DOI: 10.1113/jphysiol.2014.281253
The effects of dexamethasone on post-asphyxial cerebral oxygenation in the preterm fetal sheep
Abstract
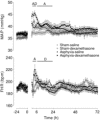
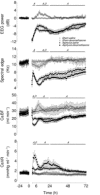
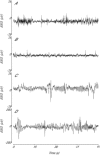
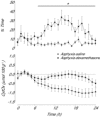
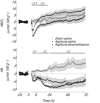
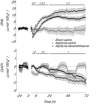
Exposure to clinical doses of the glucocorticoid dexamethasone increases brain activity and causes seizures in normoxic preterm fetal sheep without causing brain injury. In contrast, the same treatment after asphyxia increased brain injury. We hypothesised that increased injury was in part mediated by a mismatch between oxygen demand and oxygen supply. In preterm fetal sheep at 0.7 gestation we measured cerebral oxygenation using near-infrared spectroscopy, electroencephalographic (EEG) activity, and carotid blood flow (CaBF) from 24 h before until 72 h after asphyxia induced by 25 min of umbilical cord occlusion. Ewes received dexamethasone intramuscularly (12 mg 3 ml(-1)) or saline 15 min after the end of asphyxia. Fetuses were studied for 3 days after occlusion. During the first 6 h of recovery after asphyxia, dexamethasone treatment was associated with a significantly greater fall in CaBF (P < 0.05), increased carotid vascular resistance (P < 0.001) and a greater fall in cerebral oxygenation as measured by the difference between oxygenated and deoxygenated haemoglobin (delta haemoglobin; P < 0.05). EEG activity was similarly suppressed in both groups. From 6 to 10 h onward, dexamethasone treatment was associated with a return of CaBF to saline control levels, increased EEG power (P < 0.005), greater epileptiform transient activity (P < 0.001), increased oxidised cytochrome oxidase (P < 0.05) and an attenuated increase in [delta haemoglobin] (P < 0.05). In conclusion, dexamethasone treatment after asphyxia is associated with greater hypoperfusion in the critical latent phase, leading to impaired intracerebral oxygenation that may exacerbate neural injury after asphyxia.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures
References
-
- Abraham I, Juhasz G, Kekesi KA, Kovacs KJ. Effect of intrahippocampal dexamethasone on the levels of amino acid transmitters and neuronal excitability. Brain Res. 1996;733:56–63. - PubMed
-
- Bennet L, Booth LC, Drury PP, Quaedackers JS, Gunn AJ. Preterm neonatal cardiovascular instability: does understanding the fetus help evaluate the newborn. Clin Exp Pharmacol Physiol. 2012a;39:965–972. - PubMed
-
- Bennet L, Kozuma S, McGarrigle HHG, Hanson MA. Temporal changes in fetal cardiovascular, behavioural, metabolic and endocrine responses to maternally administered dexamethasone in the late gestation fetal sheep. Br J Obstet Gynaecol. 1999a;106:331–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

