A comprehensive study of myocardial redox homeostasis in naturally and mimetically aged rats
- PMID: 25384832
- PMCID: PMC4226800
- DOI: 10.1007/s11357-014-9728-y
A comprehensive study of myocardial redox homeostasis in naturally and mimetically aged rats
Abstract
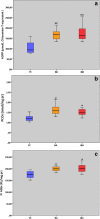
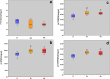
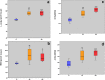
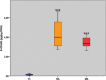
Age-related myocardial dysfunction has important implications with impaired redox homeostasis. Current study focused on investigation of redox homeostasis and histopathological changes in the myocardium of mimetically (MA), naturally aged (NA), and young control (YC) rats. Chronic D-galactose administration to young male Wistar rats (5 months old) was used to set up experimental aging models. We investigated 16 different oxidative damage biomarkers which have evaluated redox homeostasis of cellular macromolecules such as protein, lipid, and DNA. As a protein oxidation biomarker, advanced oxidation end products, protein carbonyl groups, protein-bound advanced glycation end products, dityrosine, kynurenine, and N-formylkynurenine concentrations in MA and NA rats were found to be significantly higher compared to those in YC rats. On the other hand, the levels of protein thiol groups were not significantly different between groups, whereas lipid peroxidation biomarkers such as conjugated diens, lipid hydroperoxides, and malondialdehyde in MA and NA rats were found to be significantly higher in comparison to those in YCs. For the assessment of oxidative DNA damage, we analyzed eight hydroxy-5'-deoxyguanosine concentrations of MA and NA groups which were higher than YCs. As an antioxidant status in the MA and NA groups, Cu-Zn superoxide dismutase, ferric reducing antioxidant power, and total thiol levels were lower than those in the YCs. Only nonprotein thiol levels were not significantly different. We also observed similar histopathological changes in MA and NA rats. We concluded that the mimetic aging model could be considered as a reliable experimental model for myocardial senescence.
Figures

References
-
- Babusıkova E, Kaplan P, Lehotsky J, Jesenak M, Dobrota D. Oxidative modification of rat cardiac mitochondrial membranes and myofibrils by hydroxyl radicals. Gen Physiol Biophys. 2004;23:327–335. - PubMed
-
- Babusıkova E, Jesenak M, Dobrota D, Tribulova N, Kaplan P. Age-dependent effect of oxidative stress on cardiac sarcoplasmic reticulum vesicles. Physiol Res. 2008;57:S49–S54. - PubMed
-
- Benzi IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
