Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease
- PMID: 25384911
- PMCID: PMC4341021
- DOI: 10.1007/s00262-014-1633-9
Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease
Abstract
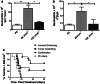
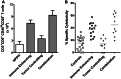
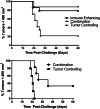
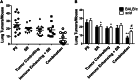
Effective therapy for advanced cancer often requires treatment of both primary tumors and systemic disease that may not be apparent at initial diagnosis. Numerous studies have shown that stimulation of the host immune system can result in the generation of anti-tumor immune responses capable of controlling metastatic tumor growth. Thus, there is interest in the development of combination therapies that both control primary tumor growth and stimulate anti-tumor immunity for control of metastatic disease and subsequent tumor growth. Photodynamic therapy (PDT) is an FDA-approved anticancer modality that has been shown to enhance anti-tumor immunity. Augmentation of anti-tumor immunity by PDT is regimen dependent, and PDT regimens that enhance anti-tumor immunity have been defined. Unfortunately, these regimens have limited ability to control primary tumor growth. Therefore, a two-step combination therapy was devised in which a tumor-controlling PDT regimen was combined with an immune-enhancing PDT regimen. To determine whether the two-step combination therapy enhanced anti-tumor immunity, resistance to subsequent tumor challenge and T cell activation and function was measured. The ability to control distant disease was also determined. The results showed that the novel combination therapy stimulated anti-tumor immunity while retaining the ability to inhibit primary tumor growth of both murine colon (Colon26-HA) and mammary (4T1) carcinomas. The combination therapy resulted in enhanced tumor-specific T cell activation and controlled metastatic tumor growth. These results suggest that PDT may be an effective adjuvant for therapies that fail to stimulate the host anti-tumor immune response.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
IL-6 potentiates tumor resistance to photodynamic therapy (PDT).Lasers Surg Med. 2011 Sep;43(7):676-85. doi: 10.1002/lsm.21107. Lasers Surg Med. 2011. PMID: 22057495 Free PMC article.
-
CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells.Br J Cancer. 2007 Jun 18;96(12):1839-48. doi: 10.1038/sj.bjc.6603792. Epub 2007 May 15. Br J Cancer. 2007. PMID: 17505510 Free PMC article.
-
Photodynamic therapy and anti-tumor immunity.Lasers Surg Med. 2006 Jun;38(5):509-15. doi: 10.1002/lsm.20362. Lasers Surg Med. 2006. PMID: 16788921
-
Photodynamic therapy: combined modality approaches targeting the tumor microenvironment.Lasers Surg Med. 2006 Jun;38(5):516-21. doi: 10.1002/lsm.20339. Lasers Surg Med. 2006. PMID: 16607618 Review.
-
Induction of tumor immunity by photodynamic therapy.J Clin Laser Med Surg. 1996 Oct;14(5):329-34. doi: 10.1089/clm.1996.14.329. J Clin Laser Med Surg. 1996. PMID: 9612200 Review.
Cited by
-
Bis[pyrrolyl Ru(ii)] triads: a new class of photosensitizers for metal-organic photodynamic therapy.Chem Sci. 2020 Oct 6;11(44):12047-12069. doi: 10.1039/d0sc04500d. eCollection 2020 Nov 28. Chem Sci. 2020. PMID: 33738086 Free PMC article.
-
Folate Receptor Targeted Photodynamic Therapy: A Novel Way to Stimulate Anti-Tumor Immune Response in Intraperitoneal Ovarian Cancer.Int J Mol Sci. 2023 Jul 10;24(14):11288. doi: 10.3390/ijms241411288. Int J Mol Sci. 2023. PMID: 37511049 Free PMC article.
-
Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies.Oncotarget. 2020 Sep 22;11(38):3531-3557. doi: 10.18632/oncotarget.27730. eCollection 2020 Sep 22. Oncotarget. 2020. PMID: 33014289 Free PMC article. Review.
-
Light-enhanced VEGF121/rGel induce immunogenic cell death and increase the antitumor activity of αCTLA4 treatment.Front Immunol. 2023 Dec 19;14:1278000. doi: 10.3389/fimmu.2023.1278000. eCollection 2023. Front Immunol. 2023. PMID: 38173721 Free PMC article.
-
Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433.Chem Rev. 2019 Jan 23;119(2):797-828. doi: 10.1021/acs.chemrev.8b00211. Epub 2018 Oct 8. Chem Rev. 2019. PMID: 30295467 Free PMC article. Review.
References
-
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

