C3d adjuvant effects are mediated through the activation of C3d-specific autoreactive T cells
- PMID: 25385064
- PMCID: PMC4323994
- DOI: 10.1038/icb.2014.89
C3d adjuvant effects are mediated through the activation of C3d-specific autoreactive T cells
Abstract
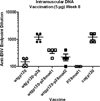
Complement fragment C3d covalently attached to antigens enhances immune responses, particularly for antigens lacking T-cell epitopes. Enhancement has been attributed to receptor cross-linking between complement receptor CR2 (CD21) and polysaccharide antigen to surface IgM on naïve B cells. Paradoxically, C3d has still been shown to increase immune responses in CD21 knockout mice, suggesting that an auxiliary activation pathway exists. In prior studies, we demonstrated the CD21-independent C3d adjuvant effect might be due to T-cell recognition of C3d T-helper epitopes processed and presented by major histocompatibility complex class II on the B-cell surface. C3d peptide sequences containing concentrated clusters of putative human C3 T-cell epitopes were identified using the epitope-mapping algorithm, EpiMatrix. These peptide sequences were synthesized and shown in vitro to bind multiple human leukocyte antigen (HLA)-DR alleles with high affinity, and induce interferon-γ responses in healthy donor peripheral blood mononuclear cells. In the present studies, we establish further correlations between HLA binding and HLA-specific lymphocyte reactions with select epitope clusters. In addition, we show that the T-cell phenotype of C3d-specific reactive T cells is CD4(+)CD45RO(+) memory T cells. Finally, mutation of a single T-cell epitope residing within the P28 peptide segment of C3d resulted in significantly diminished adjuvant activity in BALB/c mice. Collectively, these studies support the hypothesis that the paradoxical enhancement of immune responses by C3d in the absence of CD21 is due to internalization and processing of C3d into peptides that activate autoreactive CD4(+) T-helper cells in the context of HLA class II.
Conflict of interest statement
These authors recognize the presence of a potential conflict of interest and affirm that the descriptions of experiments represented in this paper are original and unbiased observations.
Figures

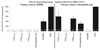
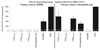
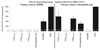
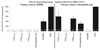
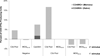

References
-
- Jakobsen H, Jonsdottir I. Mucosal vaccination against encapsulated bacteria—new potentials for conjugate vaccines? Scand J Immunol. 2003;58:119–128. - PubMed
-
- Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271:348–350. - PubMed
-
- Bower JF, Ross TM. A minimum CR2 binding domain of C3d enhances immunity following vaccination. Adv. Exp. Med. Biol. 2006;586:249–264. Adv Exp Med Biol 2006; 586: 249–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

