Pharmacokinetic interactions between primaquine and pyronaridine-artesunate in healthy adult Thai subjects
- PMID: 25385096
- PMCID: PMC4291381
- DOI: 10.1128/AAC.03829-14
Pharmacokinetic interactions between primaquine and pyronaridine-artesunate in healthy adult Thai subjects
Abstract
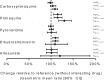
Pyronaridine-artesunate is a newly introduced artemisinin-based combination treatment which may be deployed together with primaquine. A single-dose, randomized, three-sequence crossover study was conducted in healthy Thai volunteers to characterize potential pharmacokinetic interactions between these drugs. Seventeen healthy adults received a single oral dose of primaquine alone (30 mg base) and were then randomized to receive pyronaridine-artesunate alone (540-180 mg) or pyronaridine-artesunate plus primaquine in combination, with intervening washout periods between all treatments. The pharmacokinetic properties of primaquine, its metabolite carboxyprimaquine, artesunate, its metabolite dihydroartemisinin, and pyronaridine were assessed in 15 subjects using a noncompartmental approach followed by a bioequivalence evaluation. All drugs were well tolerated. The single oral dose of primaquine did not result in any clinically relevant pharmacokinetic alterations to pyronaridine, artesunate, or dihydroartemisinin exposures. There were significantly higher primaquine maximum plasma drug concentrations (geometric mean ratio, 30%; 90% confidence interval [CI], 17% to 46%) and total exposures (15%; 6.4% to 24%) during coadministration with pyronaridine-artesunate than when primaquine was given alone. Pyronaridine, like chloroquine and piperaquine, increases plasma primaquine concentrations. (This study has been registered at ClinicalTrials.gov under registration no. NCT01552330.).
Copyright © 2015, Jittamala et al.
Figures

References
-
- World Health Organization. 2012. World malaria report 2012. World Health Organization; Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2012/en/.
-
- Duparc S, Borghini-Fuhrer I, Craft CJ, Arbe-Barnes S, Miller RM, Shin CS, Fleckenstein L. 2013. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J 12:70. doi:10.1186/1475-2875-12-70. - DOI - PMC - PubMed
-
- World Health Organization. 2010. Global report on antimalarial efficacy and drug resistance: 2000-2010. World Health Organization; Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241500470/en/.
-
- World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization; Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

