Antiviral activity of a single-domain antibody immunotoxin binding to glycoprotein D of herpes simplex virus 2
- PMID: 25385102
- PMCID: PMC4291438
- DOI: 10.1128/AAC.03818-14
Antiviral activity of a single-domain antibody immunotoxin binding to glycoprotein D of herpes simplex virus 2
Abstract
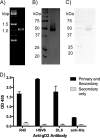
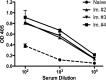
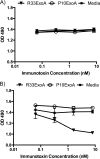
Despite years of research dedicated to preventing the sexual transmission of herpes simplex virus 2 (HSV-2), there is still no protective vaccine or microbicide against one of the most common sexually transmitted infections in the world. Using a phage display library constructed from a llama immunized with recombinant HSV-2 glycoprotein D, we identified a single-domain antibody VHH, R33, which binds to the viral surface glycoprotein D. Although R33 does not demonstrate any HSV-2 neutralization activity in vitro, when expressed with the cytotoxic domain of exotoxin A, the resulting immunotoxin (R33ExoA) specifically and potently kills HSV-2-infected cells, with a 50% neutralizing dilution (IC50) of 6.7 nM. We propose that R33ExoA could be used clinically to prevent transmission of HSV-2 through killing of virus-producing epithelial cells during virus reactivation. R33 could also potentially be used to deliver other cytotoxic effectors to HSV-2-infected cells.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
An immunotoxin targeting the gH glycoprotein of KSHV for selective killing of cells in the lytic phase of infection.Antiviral Res. 2011 Jun;90(3):143-50. doi: 10.1016/j.antiviral.2011.03.175. Epub 2011 Mar 31. Antiviral Res. 2011. PMID: 21440007 Free PMC article.
-
Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis.Nat Commun. 2015 Mar 11;6:6536. doi: 10.1038/ncomms7536. Nat Commun. 2015. PMID: 25758784 Free PMC article.
-
Recombinant CD64-specific single chain immunotoxin exhibits specific cytotoxicity against acute myeloid leukemia cells.Cancer Res. 2003 Dec 1;63(23):8414-9. Cancer Res. 2003. PMID: 14679004
-
Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin A in Cancer Therapy.Biomolecules. 2020 Jun 30;10(7):979. doi: 10.3390/biom10070979. Biomolecules. 2020. PMID: 32630017 Free PMC article. Review.
-
Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation.Front Immunol. 2020 Jun 26;11:1261. doi: 10.3389/fimmu.2020.01261. eCollection 2020. Front Immunol. 2020. PMID: 32695104 Free PMC article. Review.
Cited by
-
Research progress and applications of nanobody in human infectious diseases.Front Pharmacol. 2022 Aug 12;13:963978. doi: 10.3389/fphar.2022.963978. eCollection 2022. Front Pharmacol. 2022. PMID: 36034845 Free PMC article. Review.
-
Picomolar SARS-CoV-2 Neutralization Using Multi-Arm PEG Nanobody Constructs.Biomolecules. 2020 Dec 11;10(12):1661. doi: 10.3390/biom10121661. Biomolecules. 2020. PMID: 33322557 Free PMC article.
-
Single-Domain Antibodies and Their Formatting to Combat Viral Infections.Antibodies (Basel). 2018 Dec 20;8(1):1. doi: 10.3390/antib8010001. Antibodies (Basel). 2018. PMID: 31544807 Free PMC article. Review.
-
Single domain antibodies from camelids in the treatment of microbial infections.Front Immunol. 2024 May 17;15:1334829. doi: 10.3389/fimmu.2024.1334829. eCollection 2024. Front Immunol. 2024. PMID: 38827746 Free PMC article. Review.
-
Therapeutic applications of nanobodies against SARS-CoV-2 and other viral infections: Current update.Int J Biol Macromol. 2023 Feb 28;229:70-80. doi: 10.1016/j.ijbiomac.2022.12.284. Epub 2022 Dec 28. Int J Biol Macromol. 2023. PMID: 36586649 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

