In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122
- PMID: 25385103
- PMCID: PMC4291405
- DOI: 10.1128/AAC.04220-14
In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122
Abstract
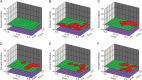
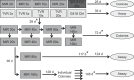
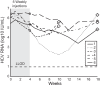
Miravirsen is a β-D-oxy-locked nucleic acid-modified phosphorothioate antisense oligonucleotide targeting the liver-specific microRNA-122 (miR-122). Miravirsen demonstrated antiviral activity against hepatitis C virus (HCV) genotype 1b replicons with a mean 50% effective concentration (EC50) of 0.67 μM. No cytotoxicity was observed up to the highest concentration tested (>320 μM) in different cell culture models, yielding a therapeutic index of ≥ 297. Combination studies of miravirsen with interferon α2b, ribavirin, and nonnucleoside (VX-222) and nucleoside (2'-methylcytidine) inhibitors of NS5B, NS5A (BMS-790052), or NS3 (telaprevir) indicated additive interactions. Miravirsen demonstrated broad antiviral activity when tested against HCV replicons resistant to NS3, NS5A, and NS5B inhibitors with less than 2-fold reductions in susceptibility. In serial passage studies, an A4C nucleotide change was observed in the HCV 5' untranslated region (UTR) from cells passaged in the presence of up to 20 μM (40-fold the miravirsen EC50 concentration) at day 72 of passage but not at earlier time points (up to 39 days of passage). Likewise, a C3U nucleotide change was observed in the HCV 5'UTR from subjects with viral rebound after the completion of therapy in a miravirsen phase 2 clinical trial. An HCV variant constructed to contain the A4C change was fully susceptible to miravirsen. A C3U HCV variant demonstrated overall reductions in susceptibility to miravirsen but was fully susceptible to all other anti-HCV agents tested. In summary, miravirsen has demonstrated broad antiviral activity and a relatively high genetic barrier to resistance. The identification of nucleotide changes associated with miravirsen resistance should help further elucidate the biology of miR-122 interactions with HCV. (The clinical trial study has been registered at ClinicalTrials.gov under registration no. NCT01200420).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Genotypic and phenotypic analyses of hepatitis C virus variants observed in clinical studies of VX-222, a nonnucleoside NS5B polymerase inhibitor.Antimicrob Agents Chemother. 2014 Sep;58(9):5456-65. doi: 10.1128/AAC.03052-14. Epub 2014 Jun 30. Antimicrob Agents Chemother. 2014. PMID: 24982088 Free PMC article. Clinical Trial.
-
Treatment of HCV infection by targeting microRNA.N Engl J Med. 2013 May 2;368(18):1685-94. doi: 10.1056/NEJMoa1209026. Epub 2013 Mar 27. N Engl J Med. 2013. PMID: 23534542 Clinical Trial.
-
Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients.Antiviral Res. 2014 Nov;111:53-9. doi: 10.1016/j.antiviral.2014.08.015. Epub 2014 Sep 8. Antiviral Res. 2014. PMID: 25218783 Clinical Trial.
-
Hepatitis C virus resistance to protease inhibitors.J Hepatol. 2011 Jul;55(1):192-206. doi: 10.1016/j.jhep.2011.01.011. Epub 2011 Feb 1. J Hepatol. 2011. PMID: 21284949 Review.
-
Future treatment of chronic hepatitis C with direct acting antivirals: is resistance important?Liver Int. 2012 Feb;32 Suppl 1:79-87. doi: 10.1111/j.1478-3231.2011.02716.x. Liver Int. 2012. PMID: 22212577 Review.
Cited by
-
Therapeutic Potential of miRNAs for Type 2 Diabetes Mellitus: An Overview.Epigenet Insights. 2022 Oct 15;15:25168657221130041. doi: 10.1177/25168657221130041. eCollection 2022. Epigenet Insights. 2022. PMID: 36262691 Free PMC article. Review.
-
Recent advances in the development and clinical application of miRNAs in infectious diseases.Noncoding RNA Res. 2024 Sep 2;10:41-54. doi: 10.1016/j.ncrna.2024.09.005. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39296638 Free PMC article. Review.
-
miRCOVID-19: Potential Targets of Human miRNAs in SARS-CoV-2 for RNA-Based Drug Discovery.Noncoding RNA. 2021 Mar 2;7(1):18. doi: 10.3390/ncrna7010018. Noncoding RNA. 2021. PMID: 33801496 Free PMC article.
-
RNA-based therapies: A cog in the wheel of lung cancer defense.Mol Cancer. 2021 Mar 19;20(1):54. doi: 10.1186/s12943-021-01338-2. Mol Cancer. 2021. PMID: 33740988 Free PMC article. Review.
-
Hepatocellular carcinoma, hepatitis C virus infection and miRNA involvement: Perspectives for new therapeutic approaches.World J Gastroenterol. 2022 Jun 14;28(22):2417-2428. doi: 10.3748/wjg.v28.i22.2417. World J Gastroenterol. 2022. PMID: 35979260 Free PMC article. Review.
References
-
- Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M, Persson A, Zhu K, Dimitrova DI, Eley T, Guo T, Grasela DM, Pasquinelli C. 2012. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 366:216–224. doi:10.1056/NEJMoa1104430. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

