Testing tuberculosis drug efficacy in a zebrafish high-throughput translational medicine screen
- PMID: 25385118
- PMCID: PMC4335901
- DOI: 10.1128/AAC.03588-14
Testing tuberculosis drug efficacy in a zebrafish high-throughput translational medicine screen
Abstract
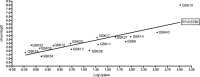
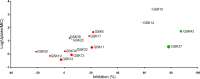
The translational value of zebrafish high-throughput screens can be improved when more knowledge is available on uptake characteristics of potential drugs. We investigated reference antibiotics and 15 preclinical compounds in a translational zebrafish-rodent screening system for tuberculosis. As a major advance, we have developed a new tool for testing drug uptake in the zebrafish model. This is important, because despite the many applications of assessing drug efficacy in zebrafish research, the current methods for measuring uptake using mass spectrometry do not take into account the possible adherence of drugs to the larval surface. Our approach combines nanoliter sampling from the yolk using a microneedle, followed by mass spectrometric analysis. To date, no single physicochemical property has been identified to accurately predict compound uptake; our method offers a great possibility to monitor how any novel compound behaves within the system. We have correlated the uptake data with high-throughput drug-screening data from Mycobacterium marinum-infected zebrafish larvae. As a result, we present an improved zebrafish larva drug-screening platform which offers new insights into drug efficacy and identifies potential false negatives and drugs that are effective in zebrafish and rodents. We demonstrate that this improved zebrafish drug-screening platform can complement conventional models of in vivo Mycobacterium tuberculosis-infected rodent assays. The detailed comparison of two vertebrate systems, fish and rodent, may give more predictive value for efficacy of drugs in humans.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
A high-throughput screen for tuberculosis progression.PLoS One. 2011 Feb 16;6(2):e16779. doi: 10.1371/journal.pone.0016779. PLoS One. 2011. PMID: 21390204 Free PMC article.
-
Screening of anti-mycobacterial compounds in a naturally infected zebrafish larvae model.J Antimicrob Chemother. 2017 Feb;72(2):421-427. doi: 10.1093/jac/dkw421. Epub 2016 Oct 24. J Antimicrob Chemother. 2017. PMID: 27798206
-
Quantification of Natural Growth of Two Strains of Mycobacterium Marinum for Translational Antituberculosis Drug Development.Clin Transl Sci. 2020 Nov;13(6):1060-1064. doi: 10.1111/cts.12793. Epub 2020 May 10. Clin Transl Sci. 2020. PMID: 32267997 Free PMC article.
-
Embryonic and larval zebrafish models for the discovery of new bioactive compounds against tuberculosis.Drug Discov Today. 2024 Nov;29(11):104163. doi: 10.1016/j.drudis.2024.104163. Epub 2024 Sep 7. Drug Discov Today. 2024. PMID: 39245344 Review.
-
The zebrafish model of tuberculosis - no lungs needed.Crit Rev Microbiol. 2018 Nov;44(6):779-792. doi: 10.1080/1040841X.2018.1523132. Epub 2019 Jan 21. Crit Rev Microbiol. 2018. PMID: 30663918 Review.
Cited by
-
An anti-tuberculosis compound screen using a zebrafish infection model identifies an aspartyl-tRNA synthetase inhibitor.Dis Model Mech. 2021 Dec 1;14(12):dmm049145. doi: 10.1242/dmm.049145. Epub 2021 Dec 23. Dis Model Mech. 2021. PMID: 34643222 Free PMC article.
-
Establishment of Infection Models in Zebrafish Larvae (Danio rerio) to Study the Pathogenesis of Aeromonas hydrophila.Front Microbiol. 2016 Aug 4;7:1219. doi: 10.3389/fmicb.2016.01219. eCollection 2016. Front Microbiol. 2016. PMID: 27540375 Free PMC article.
-
The zebrafish embryo as an in vivo model for screening nanoparticle-formulated lipophilic anti-tuberculosis compounds.Dis Model Mech. 2022 Jan 1;15(1):dmm049147. doi: 10.1242/dmm.049147. Epub 2022 Jan 26. Dis Model Mech. 2022. PMID: 34842273 Free PMC article.
-
Galleria mellonella: An Infection Model for Screening Compounds Against the Mycobacterium tuberculosis Complex.Front Microbiol. 2019 Nov 20;10:2630. doi: 10.3389/fmicb.2019.02630. eCollection 2019. Front Microbiol. 2019. PMID: 31824448 Free PMC article.
-
Zebrafish Embryo Model for Assessment of Drug Efficacy on Mycobacterial Persisters.Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00801-20. doi: 10.1128/AAC.00801-20. Print 2020 Sep 21. Antimicrob Agents Chemother. 2020. PMID: 32778551 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

