Reduction of metastatic and angiogenic potency of malignant cancer by Eupatorium fortunei via suppression of MMP-9 activity and VEGF production
- PMID: 25385232
- PMCID: PMC4227014
- DOI: 10.1038/srep06994
Reduction of metastatic and angiogenic potency of malignant cancer by Eupatorium fortunei via suppression of MMP-9 activity and VEGF production
Abstract
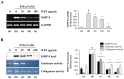
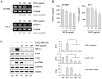
Eupatorium fortunei has long been used to treat nausea and poor appetite, and has been prescribed as a diuretic and detoxifying drug in Chinese medicine. Recent studies have demonstrated that E. fortunei possesses anti-bacterial, anti-oxidant, and anti-diabetic activities, as well as cytotoxicity to human leukemia cells. However, at non-toxic concentrations, the effects of an aqueous extract of E. fortunei (WEF) on the metastatic and angiogenic potential of malignant tumor cells have not been reported. In this study, we found that WEF suppressed the metastatic properties, including anchorage-independent colony formation, migration, and invasion, by downregulating the proteolytic activity of MMP-9. NF-κB activation and the phosphorylation of p38 and JNK were reduced significantly by WEF. Additionally, WEF inhibited tumor-induced angiogenesis markedly, affecting HUVEC migration, tube formation by HUVECs, and microvessel sprouting from rat aortic rings via a reduction in VEGF in tumors. In a pulmonary metastasis model, daily administration of WEF at 50 mg/kg markedly decreased metastatic colonies of intravenously injected B16F10 cells on the lung surface in C57BL/6J mice. Further, none of the WEF-administered mice exhibited systemic toxicity. Taken together, our results indicate that WEF is a potential therapeutic herbal product that may be useful for controlling malignant metastatic cancer.
Figures





Similar articles
-
Ethanol extract of baked Gardeniae Fructus exhibits in vitro and in vivo anti-metastatic and anti-angiogenic activities in malignant cancer cells: Role of suppression of the NF-κB and HIF-1α pathways.Int J Oncol. 2016 Dec;49(6):2377-2386. doi: 10.3892/ijo.2016.3742. Epub 2016 Oct 20. Int J Oncol. 2016. PMID: 27779658
-
Ethanol extract of Lophatheri Herba exhibits anti-cancer activity in human cancer cells by suppression of metastatic and angiogenic potential.Sci Rep. 2016 Nov 3;6:36277. doi: 10.1038/srep36277. Sci Rep. 2016. PMID: 27808120 Free PMC article.
-
Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-κB.Eur J Pharm Sci. 2012 Apr 11;45(5):581-91. doi: 10.1016/j.ejps.2011.12.012. Epub 2011 Dec 30. Eur J Pharm Sci. 2012. PMID: 22227305
-
Anti-angiogenic properties of artemisinin derivatives (Review).Int J Mol Med. 2017 Oct;40(4):972-978. doi: 10.3892/ijmm.2017.3085. Epub 2017 Jul 31. Int J Mol Med. 2017. PMID: 28765885 Review.
-
STAT3 targeting by polyphenols: Novel therapeutic strategy for melanoma.Biofactors. 2017 May 6;43(3):347-370. doi: 10.1002/biof.1345. Epub 2016 Nov 29. Biofactors. 2017. PMID: 27896891 Review.
Cited by
-
Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis.Int J Mol Sci. 2016 Jun 2;17(6):868. doi: 10.3390/ijms17060868. Int J Mol Sci. 2016. PMID: 27271600 Free PMC article. Review.
-
Long non-coding RNAs in ovarian cancer: expression profile and functional spectrum.RNA Biol. 2020 Nov;17(11):1523-1534. doi: 10.1080/15476286.2019.1702283. Epub 2019 Dec 17. RNA Biol. 2020. PMID: 31847695 Free PMC article. Review.
-
Application of scanning acoustic microscopy for evaluation of MMP activation in multiple cancer cell lines with a smart probe.Turk J Biol. 2023 Jun 5;47(3):158-169. doi: 10.55730/1300-0152.2652. eCollection 2023. Turk J Biol. 2023. PMID: 37529416 Free PMC article.
-
Quantitative Determination of p-Cymene, Thymol, Neryl Acetate, and β-Caryophyllene in Different Growth Periods and Parts of Eupatorium fortunei Turcz. by GC-MS/MS.J Anal Methods Chem. 2021 Aug 2;2021:2174667. doi: 10.1155/2021/2174667. eCollection 2021. J Anal Methods Chem. 2021. PMID: 34381625 Free PMC article.
-
Grb2-associated binder 2 silencing impairs growth and migration of H1975 cells via modulation of PI3K-Akt signaling.Int J Clin Exp Pathol. 2015 Sep 1;8(9):10575-84. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617767 Free PMC article.
References
-
- Westermarck J. & Kahari V. M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13, 781–792 (1999). - PubMed
-
- Rundhaug J. E. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res 9, 551–554 (2003). - PubMed
-
- Deryugina E. I. & Quigley J. P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25, 9–34 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

