Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury
- PMID: 25385306
- PMCID: PMC4270334
- DOI: 10.3892/mmr.2014.2905
Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury
Abstract
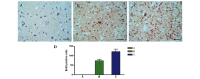
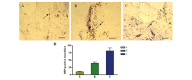
The microenvironment of the injured spinal cord is hypothesized to be involved in driving the differentiation and survival of engrafted neural stem cells (NSCs). Hypothermia is known to improve the microenvironment of the injured spinal cord in a number of ways. To investigate the effect of NSC transplantation in combination with hypothermia on the recovery of rat spinal cord injury, 60 Sprague‑Dawley female rats were used to establish a spinal cord hemisection model. They were divided randomly into three groups: A, spinal cord injury group; B, NSC transplantation group; and C, NSC transplantation + hypothermia group. At 1, 2, 4, 6 and 8 weeks post‑injury, the motor function of all animals was evaluated using the Basso, Beattie and Besnaham locomotor scoring system and the inclined plane test. At 4 weeks post‑transplantation, histological analysis and immunocytochemistry were performed. At 8 weeks post‑transplantation, horseradish peroxidase nerve tracing and transmission electron microscopy were conducted to observe axonal regeneration. The outcome of hind limb motor function recovery in group C significantly surpassed that in group B at 4 weeks post‑injury (P<0.05). Recovery was also observed in group A, but to a lesser degree. For the pathological sections no neural axonal were observed in group A. A few axon‑like structures were observed in group B and more in group C. Horseradish peroxidase‑labeled neurofibers and bromodeoxyuridine‑positive cells were observed in the spinal cords of group C. Fewer of these cells were found in group B and fewer still in group A. The differences among the three groups were significant (P<0.05). Using transmission electron microscopy, newly formed nerve fibers and myelinated nerve fibers were observed in the central transverse plane in groups B and C, although these nerve fibers were not evident in group A. In conclusion, NSC transplantation promoted the recovery of hind limb function in rats, and combination treatment with hypothermia produced synergistic effects.
Figures

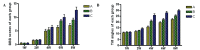


Similar articles
-
Combination of edaravone and neural stem cell transplantation repairs injured spinal cord in rats.Genet Mol Res. 2015 Dec 29;14(4):19136-43. doi: 10.4238/2015.December.29.23. Genet Mol Res. 2015. PMID: 26782566
-
Human neural stem cells promote corticospinal axons regeneration and synapse reformation in injured spinal cord of rats.Chin Med J (Engl). 2006 Aug 20;119(16):1331-8. Chin Med J (Engl). 2006. PMID: 16934177
-
[TRANSPLANTATION OF NEURAL STEM CELLS INDUCED BY ALL-TRANS- RETINOIC ACID COMBINED WITH GLIAL CELL LINE DERIVED NEUROTROPHIC FACTOR AND CHONDROITINASE ABC FOR REPAIRING SPINAL CORD INJURY OF RATS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015 Aug;29(8):1009-15. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015. PMID: 26677625 Chinese.
-
Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys?Stem Cells. 2015 Apr;33(4):1036-41. doi: 10.1002/stem.1959. Stem Cells. 2015. PMID: 25728093 Review.
-
Transplantation of neural stem cells into the spinal cord after injury.Semin Cell Dev Biol. 2003 Jun;14(3):191-8. doi: 10.1016/s1084-9521(03)00011-9. Semin Cell Dev Biol. 2003. PMID: 12948354 Review.
Cited by
-
Neural stem cell transplantation inhibits glial cell proliferation and P2X receptor-mediated neuropathic pain in spinal cord injury rats.Neural Regen Res. 2019 May;14(5):876-885. doi: 10.4103/1673-5374.249236. Neural Regen Res. 2019. PMID: 30688274 Free PMC article.
-
Hypothermic treatment after computer-controlled compression in minipig: A preliminary report on the effect of epidural vs. direct spinal cord cooling.Exp Ther Med. 2018 Dec;16(6):4927-4942. doi: 10.3892/etm.2018.6831. Epub 2018 Oct 5. Exp Ther Med. 2018. PMID: 30542449 Free PMC article.
-
Efficacy of neural stem cell transplantation for the treatment of patients with spinal cord injury: A protocol of systematic review and meta-analysis.Medicine (Baltimore). 2020 May;99(19):e20169. doi: 10.1097/MD.0000000000020169. Medicine (Baltimore). 2020. PMID: 32384508 Free PMC article.
-
Mild hypothermia enhances regenerative gene expression in late-stage neural precursors.Med Int (Lond). 2025 Jul 16;5(5):53. doi: 10.3892/mi.2025.252. eCollection 2025 Sep-Oct. Med Int (Lond). 2025. PMID: 40747152 Free PMC article.
-
Therapeutic Hypothermia in Spinal Cord Injury: The Status of Its Use and Open Questions.Int J Mol Sci. 2015 Jul 24;16(8):16848-79. doi: 10.3390/ijms160816848. Int J Mol Sci. 2015. PMID: 26213924 Free PMC article. Review.
References
-
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

