Insulin-like growth factor-I inhibition with pasireotide decreases cell proliferation and increases apoptosis in pre-malignant lesions of the breast: a phase 1 proof of principle trial
- PMID: 25385439
- PMCID: PMC4303192
- DOI: 10.1186/s13058-014-0463-1
Insulin-like growth factor-I inhibition with pasireotide decreases cell proliferation and increases apoptosis in pre-malignant lesions of the breast: a phase 1 proof of principle trial
Abstract
Introduction: Estrogen inhibition is effective in preventing breast cancer in only up to 50% of women with precancerous lesions and many experience side effects that are poorly tolerated. As insulin-like growth factor I (IGF-I) underlies both estrogen and progesterone actions and has other direct effects on mammary development and carcinogenesis, we hypothesized that IGF-I inhibition might provide a novel approach for breast cancer chemoprevention.
Methods: In total, 13 women with core breast biopsies diagnostic of atypical hyperplasia (AH) were treated for 10 days with pasireotide, a somatostatin analog which uniquely inhibits IGF-I action in the mammary gland. They then had excision biopsies. 12 patients also had proliferative lesions and one a ductal carcinoma in situ (DCIS). Primary outcomes were changes in cell proliferation and apoptosis after treatment. Expression of estrogen receptor (ER), progesterone receptor (PR), and phosphorylated Insulin-like growth factor I receptor (IGF-1R), protein kinase B (AKT) and extracellular signal-regulated kinases 1/2 (ERK1/2) were also assessed. Core and excision biopsies from 14 untreated patients served as non-blinded controls. Hyperglycemia and other side effects were carefully monitored.
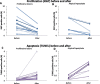
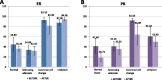
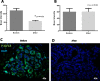
Results: Pasireotide decreased proliferation and increased apoptosis in all AH (from 3.6 ± 2.6% to 1.3 ± 1.2% and from 0.3 ± 0.2% to 1.5 ± 1.6%, respectively) and proliferative lesions (from 3.8 ± 2.5% to 1.8 ± 1.8% and from 0.3 ± 0.2% to 1.3 ± 0.6%, respectively). The DCIS responded similarly. ER and PR were not affected by pasireotide, while IGF-1R, ERK1/2 and AKT phosphorylation decreased significantly. In contrast, tissue from untreated controls showed no change in cell proliferation or phosphorylation of IGF-1R, AKT or ERK 1/2. Mild to moderate hyperglycemia associated with reduced insulin levels was found. Glucose fell into the normal range after discontinuing treatment. Pasireotide was well tolerated and did not cause symptoms of estrogen deprivation.
Conclusions: IGF-I inhibition by pasireotide, acting through the IGF-1R, was associated with decreased proliferation and increased apoptosis in pre-malignant breast lesions and one DCIS. Assuming hyperglycemia can be controlled, these data suggest that inhibiting the IGF-I pathway may prove an effective alternative for breast cancer chemoprevention.
Trial registration: NCT01372644 Trial date: July 1, 2007.
Figures


Similar articles
-
Insulin-like growth factor receptor-1 (IGF-1R) expression in normal breast, proliferative breast lesions, and breast carcinoma.Appl Immunohistochem Mol Morphol. 2011 May;19(3):218-25. doi: 10.1097/PAI.0b013e3181ffc58c. Appl Immunohistochem Mol Morphol. 2011. PMID: 21217522
-
Pasireotide, an IGF-I action inhibitor, prevents growth hormone and estradiol-induced mammary hyperplasia.Pituitary. 2011 Mar;14(1):44-52. doi: 10.1007/s11102-010-0257-0. Pituitary. 2011. PMID: 20890664
-
Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation.Breast Cancer Res. 2013;15(4):R55. doi: 10.1186/bcr3449. Breast Cancer Res. 2013. PMID: 23844554 Free PMC article.
-
Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions.Endocr Rev. 2009 Feb;30(1):51-74. doi: 10.1210/er.2008-0022. Epub 2008 Dec 15. Endocr Rev. 2009. PMID: 19075184 Free PMC article. Review.
-
Atypical lobular hyperplasia and lobular carcinoma in situ: surgical and molecular pathology.Histopathology. 1999 Sep;35(3):195-200. doi: 10.1046/j.1365-2559.1999.00815.x. Histopathology. 1999. PMID: 10469210 Review.
Cited by
-
Lack of cortistatin or somatostatin differentially influences DMBA-induced mammary gland tumorigenesis in mice in an obesity-dependent mode.Breast Cancer Res. 2016 Mar 8;18(1):29. doi: 10.1186/s13058-016-0689-1. Breast Cancer Res. 2016. PMID: 26956474 Free PMC article.
-
Understanding Susceptibility to Breast Cancer: From Risk Factors to Prevention Strategies.Int J Mol Sci. 2025 Mar 25;26(7):2993. doi: 10.3390/ijms26072993. Int J Mol Sci. 2025. PMID: 40243654 Free PMC article. Review.
-
RCC2 Expression Stimulates ER-Positive Breast Tumorigenesis.J Oncol. 2020 May 23;2020:5619462. doi: 10.1155/2020/5619462. eCollection 2020. J Oncol. 2020. PMID: 32565805 Free PMC article.
-
Phase I dose-escalation study of long-acting pasireotide in patients with neuroendocrine tumors.Onco Targets Ther. 2017 Jun 27;10:3177-3186. doi: 10.2147/OTT.S128547. eCollection 2017. Onco Targets Ther. 2017. PMID: 28721067 Free PMC article.
-
Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9302. doi: 10.3390/ijms25179302. Int J Mol Sci. 2024. PMID: 39273251 Free PMC article. Review.
References
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. - DOI - PubMed
-
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. - DOI - PubMed
-
- Coopey SB, Mazzola E, Buckley JM, Sharko J, Belli AK, Kim EM, Polubriaginof F, Parmigiani G, Garber JE, Smith BL, Gadd MA, Specht MC, Guidi AJ, Roche CA, Hughes KS. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136:627–633. doi: 10.1007/s10549-012-2318-8. - DOI - PubMed
-
- Ruan W, Catanese V, Wieczorek R, Feldman M, Kleinberg DL. Estradiol enhances the stimulatory effect of insulin-like growth factor-I (IGF-I) on mammary development and growth hormone-induced IGF-I messenger ribonucleic acid. Endocrinology. 1995;136:1296–1302. doi: 10.1210/endo.136.3.7867584. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

