Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs
- PMID: 25385584
- PMCID: PMC4250103
- DOI: 10.1073/pnas.1406997111
Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs
Abstract
The cardiovascular safety of nonsteroidal antiinflammatory drugs (NSAIDs) may be influenced by interactions with antiplatelet doses of aspirin. We sought to quantitate precisely the propensity of commonly consumed NSAIDs—ibuprofen, naproxen, and celecoxib—to cause a drug-drug interaction with aspirin in vivo by measuring the target engagement of aspirin directly by MS. We developed a novel assay of cyclooxygenase-1 (COX-1) acetylation in platelets isolated from volunteers who were administered aspirin and used conventional and microfluidic assays to evaluate platelet function. Although ibuprofen, naproxen, and celecoxib all had the potential to compete with the access of aspirin to the substrate binding channel of COX-1 in vitro, exposure of volunteers to a single therapeutic dose of each NSAID followed by 325 mg aspirin revealed a potent drug-drug interaction between ibuprofen and aspirin and between naproxen and aspirin but not between celecoxib and aspirin. The imprecision of estimates of aspirin consumption and the differential impact on the ability of aspirin to inactivate platelet COX-1 will confound head-to-head comparisons of distinct NSAIDs in ongoing clinical studies designed to measure their cardiovascular risk.
Keywords: MS; acetylation; aspirin; cyclooxygenase; nonsteroidal antiinflammatory drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
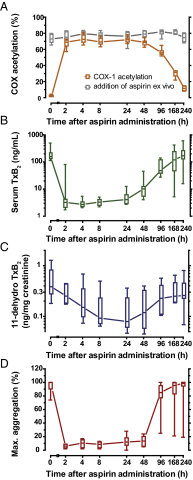
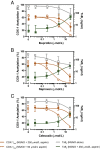

References
-
- Institute of Medicine . Relieving Pain in America. A Blueprint for Transforming Prevention, Care, Education and Research. National Academy Press; Washington, DC: 2011. - PubMed
-
- Grosser T, Yu Y, FitzGerald GA. Emotion recollected in tranquility: Lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

