Sample processing obscures cancer-specific alterations in leukemic transcriptomes
- PMID: 25385641
- PMCID: PMC4250124
- DOI: 10.1073/pnas.1413374111
Sample processing obscures cancer-specific alterations in leukemic transcriptomes
Abstract
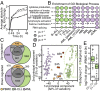
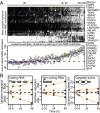
Substantial effort is currently devoted to identifying cancer-associated alterations using genomics. Here, we show that standard blood collection procedures rapidly change the transcriptional and posttranscriptional landscapes of hematopoietic cells, resulting in biased activation of specific biological pathways; up-regulation of pseudogenes, antisense RNAs, and unannotated coding isoforms; and RNA surveillance inhibition. Affected genes include common mutational targets and thousands of other genes participating in processes such as chromatin modification, RNA splicing, T- and B-cell activation, and NF-κB signaling. The majority of published leukemic transcriptomes exhibit signals of this incubation-induced dysregulation, explaining up to 40% of differences in gene expression and alternative splicing between leukemias and reference normal transcriptomes. The effects of sample processing are particularly evident in pan-cancer analyses. We provide biomarkers that detect prolonged incubation of individual samples and show that keeping blood on ice markedly reduces changes to the transcriptome. In addition to highlighting the potentially confounding effects of technical artifacts in cancer genomics data, our study emphasizes the need to survey the diversity of normal as well as neoplastic cells when characterizing tumors.
Keywords: RNA splicing; batch effects; leukemia; nonsense-mediated decay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

