RGC32 deficiency protects against high-fat diet-induced obesity and insulin resistance in mice
- PMID: 25385871
- PMCID: PMC4293277
- DOI: 10.1530/JOE-14-0548
RGC32 deficiency protects against high-fat diet-induced obesity and insulin resistance in mice
Abstract
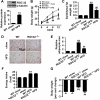
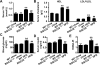
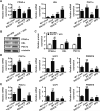
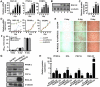
Obesity is an important independent risk factor for type 2 diabetes, cardiovascular diseases and many other chronic diseases. Adipose tissue inflammation is a critical link between obesity and insulin resistance and type 2 diabetes and a contributor to disease susceptibility and progression. The objective of this study was to determine the role of response gene to complement 32 (RGC32) in the development of obesity and insulin resistance. WT and RGC32 knockout (Rgc32(-/-) (Rgcc)) mice were fed normal chow or high-fat diet (HFD) for 12 weeks. Metabolic, biochemical, and histologic analyses were performed. 3T3-L1 preadipocytes were used to study the role of RGC32 in adipocytes in vitro. Rgc32(-/-) mice fed with HFD exhibited a lean phenotype with reduced epididymal fat weight compared with WT controls. Blood biochemical analysis and insulin tolerance test showed that RGC32 deficiency improved HFD-induced dyslipidemia and insulin resistance. Although it had no effect on adipocyte differentiation, RGC32 deficiency ameliorated adipose tissue and systemic inflammation. Moreover, Rgc32(-/-) induced browning of adipose tissues and increased energy expenditure. Our data indicated that RGC32 plays an important role in diet-induced obesity and insulin resistance, and thus it may serve as a potential novel drug target for developing therapeutics to treat obesity and metabolic disorders.
Keywords: adipose tissue; insulin resistance; obesity; response gene to complement 32.
© 2015 Society for Endocrinology.
Figures
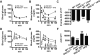
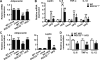
References
-
- Badea TC, Niculescu FI, Soane L, Shin ML, Rus H. Molecular cloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J Biol Chem. 1998;273:26977–26981. - PubMed
-
- Bandyopadhyay P. The role of incretin-based therapies in the management of type 2 diabetes. Drug News Perspect. 2009;22:559–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

