Hypoxia and fatty liver
- PMID: 25386057
- PMCID: PMC4223242
- DOI: 10.3748/wjg.v20.i41.15087
Hypoxia and fatty liver
Abstract
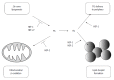
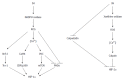
The liver is a central organ that metabolizes excessive nutrients for storage in the form of glycogen and lipids and supplies energy-producing substrates to the peripheral tissues to maintain their function, even under starved conditions. These processes require a considerable amount of oxygen, which causes a steep oxygen gradient throughout the hepatic lobules. Alcohol consumption and/or excessive food intake can alter the hepatic metabolic balance drastically, which can precipitate fatty liver disease, a major cause of chronic liver diseases worldwide, ranging from simple steatosis, through steatohepatitis and hepatic fibrosis, to liver cirrhosis. Altered hepatic metabolism and tissue remodeling in fatty liver disease further disrupt hepatic oxygen homeostasis, resulting in severe liver hypoxia. As master regulators of adaptive responses to hypoxic stress, hypoxia-inducible factors (HIFs) modulate various cellular and organ functions, including erythropoiesis, angiogenesis, metabolic demand, and cell survival, by activating their target genes during fetal development and also in many disease conditions such as cancer, heart failure, and diabetes. In the past decade, it has become clear that HIFs serve as key factors in the regulation of lipid metabolism and fatty liver formation. This review discusses the molecular mechanisms by which hypoxia and HIFs regulate lipid metabolism in the development and progression of fatty liver disease.
Keywords: Fatty liver disease; Hypoxia; Hypoxia-inducible factor; Lipid metabolism; Obstructive sleep apnea.
Figures

References
-
- Savage DB, Semple RK. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol. 2010;21:329–336. - PubMed
-
- Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. - PubMed
-
- Crabb DW, Liangpunsakul S. Alcohol and lipid metabolism. J Gastroenterol Hepatol. 2006;21 Suppl 3:S56–S60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

