Central gain control in tinnitus and hyperacusis
- PMID: 25386157
- PMCID: PMC4208401
- DOI: 10.3389/fneur.2014.00206
Central gain control in tinnitus and hyperacusis
Abstract
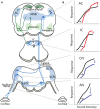
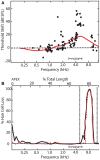
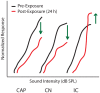
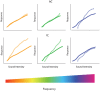
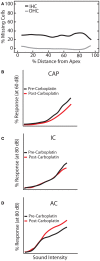
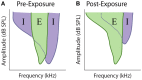
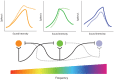
Sensorineural hearing loss induced by noise or ototoxic drug exposure reduces the neural activity transmitted from the cochlea to the central auditory system. Despite a reduced cochlear output, neural activity from more central auditory structures is paradoxically enhanced at suprathreshold intensities. This compensatory increase in the central auditory activity in response to the loss of sensory input is referred to as central gain enhancement. Enhanced central gain is hypothesized to be a potential mechanism that gives rise to hyperacusis and tinnitus, two debilitating auditory perceptual disorders that afflict millions of individuals. This review will examine the evidence for gain enhancement in the central auditory system in response to cochlear damage. Further, it will address the potential cellular and molecular mechanisms underlying this enhancement and discuss the contribution of central gain enhancement to tinnitus and hyperacusis. Current evidence suggests that multiple mechanisms with distinct temporal and spectral profiles are likely to contribute to central gain enhancement. Dissecting the contributions of these different mechanisms at different levels of the central auditory system is essential for elucidating the role of central gain enhancement in tinnitus and hyperacusis and, most importantly, the development of novel treatments for these disorders.
Keywords: central gain enhancement; homeostatic plasticity; hyperacusis; lateral inhibition; tinnitus.
Figures

References
-
- Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow J, editor. Tinnitus Theory and Management. Hamilton, ON: BC Decker; (2004). p. 16–41
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

