Eosinophil secretion of granule-derived cytokines
- PMID: 25386174
- PMCID: PMC4209865
- DOI: 10.3389/fimmu.2014.00496
Eosinophil secretion of granule-derived cytokines
Abstract
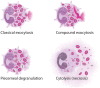
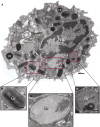
Eosinophils are tissue-dwelling leukocytes, present in the thymus, and gastrointestinal and genitourinary tracts of healthy individuals at baseline, and recruited, often in large numbers, to allergic inflammatory foci and sites of active tissue repair. The biological significance of eosinophils is vast and varied. In health, eosinophils support uterine and mammary gland development, and maintain bone marrow plasma cells and adipose tissue alternatively activated macrophages, while in response to tissue insult eosinophils function as inflammatory effector cells, and, in the wake of an inflammatory response, promote tissue regeneration, and wound healing. One common mechanism driving many of the diverse eosinophil functions is the regulated and differential secretion of a vast array of eosinophil-derived cytokines. Eosinophils are distinguished from most other leukocytes in that many, if not all, of the over three dozen eosinophil-derived cytokines are pre-synthesized and stored within intracellular granules, poised for very rapid, stimulus-induced secretion. Eosinophils engaged in cytokine secretion in situ utilize distinct pathways of cytokine release that include classical exocytosis, whereby granules themselves fuse with the plasma membrane and release their entire contents extracellularly; piecemeal degranulation, whereby granule-derived cytokines are selectively mobilized into vesicles that emerge from granules, traverse the cytoplasm and fuse with the plasma membrane to release discrete packets of cytokines; and eosinophil cytolysis, whereby intact granules are extruded from eosinophils, and deposited within tissues. In this latter scenario, extracellular granules can themselves function as stimulus-responsive secretory-competent organelles within the tissue. Here, we review the distinctive processes of differential secretion of eosinophil granule-derived cytokines.
Keywords: cytokine; cytolysis; degranulation; eosinophil; granule; piecemeal degranulation; secretion.
Figures



References
-
- Spry CJF. Eosinophils: A Comprehensive Review and Guide to the Scientific and Medical Literature. Oxford: Oxford Medical Publications; (1988).
-
- Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem (1993) 41(11):1611–5.10.1177/41.11.8409368 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

