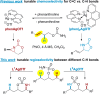
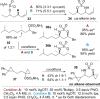
Ligand-controlled, tunable silver-catalyzed C-H amination
- PMID: 25386769
- PMCID: PMC4277762
- DOI: 10.1021/ja5094309
Ligand-controlled, tunable silver-catalyzed C-H amination
Abstract
The development of readily tunable and regioselective C-H functionalization reactions that operate solely through catalyst control remains a challenge in modern organic synthesis. Herein, we report that simple silver catalysts supported by common nitrogenated ligands can be used to tune a nitrene transfer reaction between two different types of C-H bonds. The results reported herein represent the first example of ligand-controlled and site-selective silver-promoted C-H amination.
Figures
References
-
-
For selected references on selective C–H functionalizations, see:
- Roizen J. L.; Harvey M. E.; Du Bois J. Acc. Chem. Res. 2012, 45, 911. - PMC - PubMed
- Lescot C.; Darses B.; Collet F.; Retailleau P.; Dauban P. J. Org. Chem. 2012, 77, 7232. - PubMed
- Newhouse T.; Baran P. S. Angew. Chem., Int. Ed. 2011, 50, 3362. - PMC - PubMed
- Collet F.; Lescot C.; Liang C.; Dauban P. Dalton Trans. 2010, 39, 10401. - PubMed
- Bess E. N.; DeLuca R. J.; Tindall D. J.; Oderinde M. S.; Roizen J. L.; Du Bois J.; Sigman M. S. J. Am. Chem. Soc. 2014, 136, 5783. - PMC - PubMed
- Chen M. S.; White M. C. Science 2007, 318, 783. - PubMed
- Chen M. S.; White M. C. Science 2010, 327, 566. - PubMed
- White M. C. Science 2012, 335, 807. - PubMed
- Bagchi V.; Paraskevopoulou P.; Das P.; Chi L.; Wang Q.; Choudhury A.; Mathieson J. S.; Cronin L.; Pardue D. B.; Cundari T. R.; Mitrikas G.; Sanakis Y.; Stavropoulos P. J. Am. Chem. Soc. 2014, 136, 11362. - PubMed
-
-
-
For selected examples of directing groups used in conjunction with Ir catalysts, see:
- Kim H. J.; Ajitha M. J.; Ryu J.; Kim J.; Lee Y.; Jung Y.; Chang S. J. Am. Chem. Soc. 2014, 136, 1132. - PubMed
- Kang T.; Kim Y.; Lee D.; Wang Z.; Chang S. J. Am. Chem. Soc. 2014, 136, 4141. - PubMed
- Simmons E. M.; Hartwig J. F. Nature 2012, 483, 71. - PMC - PubMed
-
For examples involving Pd, see:
- Shao J.; Chen W.; Giulianotti M. A.; Houghten R. A.; Yu Y. Org. Lett. 2012, 14, 5452. - PMC - PubMed
- Musaev D. G.; Kaledin A.; Shi B.; Yu J. J. Am. Chem. Soc. 2012, 134, 1690. - PubMed
- Desai L. V.; Stowers K. J.; Sanford M. S. J. Am. Chem. Soc. 2008, 130, 13285. - PMC - PubMed
-
For examples involving Ru, see:
- Allu S.; Swarmy K. C. K. J. Org. Chem. 2014, 79, 3963. - PubMed
-
-
- Espino C. G.; Wehn P. M.; Chow J.; Du Bois J. J. Am. Chem. Soc. 2001, 123, 6935.
- Collet F.; Dodd R. H.; Dauban P. Chem. Commun. 2009, 5061. - PubMed
- Fiori K. W.; Espino C. G.; Brodsky B. H.; Du Bois J. Tetrahedron 2009, 65, 3042.
- Norder A.; Warren S. A.; Herdtweck E.; Huber S. M.; Bach T. J. Am. Chem. Soc. 2012, 134, 13524. - PubMed
-
-
For other examples of Rh-catalyzed C–H amination, see:
- Lescot C.; Darses B.; Collet C.; Retailleau P.; Dauban P. J. Org. Chem. 2012, 77, 7232. - PubMed
- Cochet T.; Bellosta V.; Roche D.; Ortholand J.; Greiner A.; Cossy J. Chem. Commun. 2012, 48, 10745. - PubMed
- Grohmann C.; Wang H.; Glorius F. Org. Lett. 2012, 14, 656. - PubMed
- Ng K.; Zhou Z.; Yu W. Org. Lett. 2012, 14, 272. - PubMed
- Lebel H.; Spitz C.; Leogane O.; Trudel C.; Parmentier M. Org. Lett. 2011, 13, 5460. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources