Advanced glycation endproducts interfere with adhesion and neurite outgrowth
- PMID: 25386903
- PMCID: PMC4227844
- DOI: 10.1371/journal.pone.0112115
Advanced glycation endproducts interfere with adhesion and neurite outgrowth
Abstract
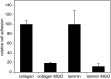
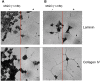
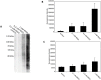
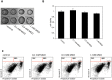
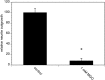
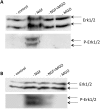
Advanced glycation endproducts (AGEs) represent a non-enzymatic posttranslational protein modification. AGEs are generated by a series of chemical reactions of free reducing monosaccharides, such as glucose, fructose or metabolites of the monosaccharide metabolism with amino groups of proteins. After oxidation, dehydration and condensation, stable AGE-modifications are formed. AGE-modified proteins accumulate in all cells and tissues as a normal feature of ageing and correlate with the glucose concentration in the blood. AGEs are increased in diabetic patients and play a significant role in the pathogenesis of most age-related neural disorders, such as Alzheimer's disease. We examined the role of AGEs on neurite outgrowth of PC12 cells. We induced the formation of AGEs using the reactive carbonyl compound methylglyoxal (MGO) as a physiological metabolite of glucose. We found that AGE-modification of laminin or collagen interfered with adhesion but not with neurite outgrowth of PC12 cells. Furthermore, the AGE-modification of PC12 cell proteins reduced NGF-induced neurite outgrowth. In conclusion, our data show that AGEs negatively influence neural plasticity.
Conflict of interest statement
Figures


References
-
- Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: A review. Diabetologia 44: 129–146. - PubMed
-
- Requena V, Villena A, Díaz F, González F, Ríus F, et al. (1996) The effect of ageing on neurons in the visual sector of the thalamic reticular nucleus. Mech Ageing Dev 89: 185–193. - PubMed
-
- Krautwald M, Münch G (2010) Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease?. Exp Gerontol 45: 744–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

