Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances
- PMID: 25387163
- PMCID: PMC4284462
- DOI: 10.3390/life4040666
Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances
Abstract
Ferredoxins (Fed), occurring in most organisms, are small proteins that use their iron-sulfur cluster to distribute electrons to various metabolic pathways, likely including hydrogen production. Here, we summarize the current knowledge on ferredoxins in cyanobacteria, the prokaryotes regarded as important producers of the oxygenic atmosphere and biomass for the food chain, as well as promising cell factories for biofuel production. Most studies of ferredoxins were performed in the model strain, Synechocystis PCC6803, which possesses nine highly-conserved ferredoxins encoded by monocistronic or operonic genes, some of which are localized in conserved genome regions. Fed1, encoded by a light-inducible gene, is a highly abundant protein essential to photosynthesis. Fed2-Fed9, encoded by genes differently regulated by trophic conditions, are low-abundant proteins that play prominent roles in the tolerance to environmental stresses. Concerning the selectivity/redundancy of ferredoxin, we report that Fed1, Fed7 and Fed9 belong to ferredoxin-glutaredoxin-thioredoxin crosstalk pathways operating in the protection against oxidative and metal stresses. Furthermore, Fed7 specifically interacts with a DnaJ-like protein, an interaction that has been conserved in photosynthetic eukaryotes in the form of a composite protein comprising DnaJ- and Fed7-like domains. Fed9 specifically interacts with the Flv3 flavodiiron protein acting in the photoreduction of O2 to H2O.
Figures


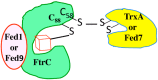
References
-
- Sticht H., Rosch P. The structure of iron-sulfur proteins. Prog. Biophys. Mol. Biol. 1998;70:95–136. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

