Modulatory effects of taurine on jejunal contractility
- PMID: 25387674
- PMCID: PMC4244673
- DOI: 10.1590/1414-431X20142890
Modulatory effects of taurine on jejunal contractility
Abstract
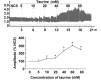
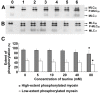
Taurine (2-aminoethanesulfonic acid) is widely distributed in animal tissues and has diverse pharmacological effects. However, the role of taurine in modulating smooth muscle contractility is still controversial. We propose that taurine (5-80 mM) can exert bidirectional modulation on the contractility of isolated rat jejunal segments. Different low and high contractile states were induced in isolated jejunal segments of rats to observe the effects of taurine and the associated mechanisms. Taurine induced stimulatory effects on the contractility of isolated rat jejunal segments at 3 different low contractile states, and inhibitory effects at 3 different high contractile states. Bidirectional modulation was not observed in the presence of verapamil or tetrodotoxin, suggesting that taurine-induced bidirectional modulation is Ca(2+) dependent and requires the presence of the enteric nervous system. The stimulatory effects of taurine on the contractility of isolated jejunal segments was blocked by atropine but not by diphenhydramine or by cimetidine, suggesting that muscarinic-linked activation was involved in the stimulatory effects when isolated jejunal segments were in a low contractile state. The inhibitory effects of taurine on the contractility of isolated jejunal segments were blocked by propranolol and L-NG-nitroarginine but not by phentolamine, suggesting that adrenergic β receptors and a nitric oxide relaxing mechanism were involved when isolated jejunal segments were in high contractile states. No bidirectional effects of taurine on myosin phosphorylation were observed. The contractile states of jejunal segments determine taurine-induced stimulatory or inhibitory effects, which are associated with muscarinic receptors and adrenergic β receptors, and a nitric oxide associated relaxing mechanism.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

