Barley beta-glucan promotes MnSOD expression and enhances angiogenesis under oxidative microenvironment
- PMID: 25388628
- PMCID: PMC4288365
- DOI: 10.1111/jcmm.12442
Barley beta-glucan promotes MnSOD expression and enhances angiogenesis under oxidative microenvironment
Abstract
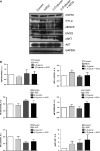
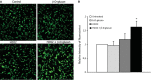
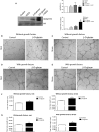
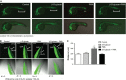
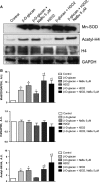
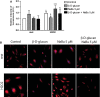
Manganese superoxide dismutase (MnSOD), a foremost antioxidant enzyme, plays a key role in angiogenesis. Barley-derived (1.3) β-d-glucan (β-d-glucan) is a natural water-soluble polysaccharide with antioxidant properties. To explore the effects of β-d-glucan on MnSOD-related angiogenesis under oxidative stress, we tested epigenetic mechanisms underlying modulation of MnSOD level in human umbilical vein endothelial cells (HUVECs) and angiogenesis in vitro and in vivo. Long-term treatment of HUVECs with 3% w/v β-d-glucan significantly increased the level of MnSOD by 200% ± 2% compared to control and by 50% ± 4% compared to untreated H2 O2 -stressed cells. β-d-glucan-treated HUVECs displayed greater angiogenic ability. In vivo, 24 hrs-treatment with 3% w/v β-d-glucan rescued vasculogenesis in Tg (kdrl: EGFP) s843Tg zebrafish embryos exposed to oxidative microenvironment. HUVECs overexpressing MnSOD demonstrated an increased activity of endothelial nitric oxide synthase (eNOS), reduced load of superoxide anion (O2 (-) ) and an increased survival under oxidative stress. In addition, β-d-glucan prevented the rise of hypoxia inducible factor (HIF)1-α under oxidative stress. The level of histone H4 acetylation was significantly increased by β-d-glucan. Increasing histone acetylation by sodium butyrate, an inhibitor of class I histone deacetylases (HDACs I), did not activate MnSOD-related angiogenesis and did not impair β-d-glucan effects. In conclusion, 3% w/v β-d-glucan activates endothelial expression of MnSOD independent of histone acetylation level, thereby leading to adequate removal of O2 (-) , cell survival and angiogenic response to oxidative stress. The identification of dietary β-d-glucan as activator of MnSOD-related angiogenesis might lead to the development of nutritional approaches for the prevention of ischemic remodelling and heart failure.
Keywords: angiogenesis; antioxidants; beta-glucan; endothelial cells; histone deacetylases.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Yeast-derived particulate beta-glucan induced angiogenesis via regulating PI3K/Src and ERK1/2 signaling pathway.Int J Biol Macromol. 2024 Jun;269(Pt 2):131884. doi: 10.1016/j.ijbiomac.2024.131884. Epub 2024 Apr 27. Int J Biol Macromol. 2024. PMID: 38685541
-
Ionic silicon improves endothelial cells' survival under toxic oxidative stress by overexpressing angiogenic markers and antioxidant enzymes.J Tissue Eng Regen Med. 2018 Nov;12(11):2203-2220. doi: 10.1002/term.2744. Epub 2018 Oct 24. J Tissue Eng Regen Med. 2018. PMID: 30062712 Free PMC article.
-
Spatholobi Caulis extracts promote angiogenesis in HUVECs in vitro and in zebrafish embryos in vivo via up-regulation of VEGFRs.J Ethnopharmacol. 2017 Mar 22;200:74-83. doi: 10.1016/j.jep.2016.10.075. Epub 2016 Oct 27. J Ethnopharmacol. 2017. PMID: 27989880
-
Insights into Manganese Superoxide Dismutase and Human Diseases.Int J Mol Sci. 2022 Dec 14;23(24):15893. doi: 10.3390/ijms232415893. Int J Mol Sci. 2022. PMID: 36555531 Free PMC article. Review.
-
Modulation of MnSOD in Cancer:Epidemiological and Experimental Evidence.Toxicol Res. 2010 Jun;26(2):83-93. doi: 10.5487/TR.2010.26.2.083. Toxicol Res. 2010. PMID: 24278510 Free PMC article. Review.
Cited by
-
The role of polysaccharide peptide of Ganoderma lucidum as a potent antioxidant against atherosclerosis in high risk and stable angina patients.Indian Heart J. 2018 Sep-Oct;70(5):608-614. doi: 10.1016/j.ihj.2017.12.007. Epub 2017 Dec 14. Indian Heart J. 2018. PMID: 30392496 Free PMC article. Clinical Trial.
-
Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways.J Cell Mol Med. 2020 Nov;24(21):12355-12367. doi: 10.1111/jcmm.15725. Epub 2020 Sep 22. J Cell Mol Med. 2020. PMID: 32961025 Free PMC article.
-
Extracellular Vesicles Derived From Human Umbilical Cord Mesenchymal Stem Cells Protect Against DOX-Induced Heart Failure Through the miR-100-5p/NOX4 Pathway.Front Bioeng Biotechnol. 2021 Aug 25;9:703241. doi: 10.3389/fbioe.2021.703241. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34513812 Free PMC article.
-
Sinapic Acid Inhibits Cardiac Hypertrophy via Activation of Mitochondrial Sirt3/SOD2 Signaling in Neonatal Rat Cardiomyocytes.Antioxidants (Basel). 2020 Nov 21;9(11):1163. doi: 10.3390/antiox9111163. Antioxidants (Basel). 2020. PMID: 33233476 Free PMC article.
-
Novel Insight Into the Epigenetic and Post-transcriptional Control of Cardiac Gene Expression by Thyroid Hormone.Front Endocrinol (Lausanne). 2019 Aug 29;10:601. doi: 10.3389/fendo.2019.00601. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31555215 Free PMC article. Review.
References
-
- Higashi Y, Maruhashi T, Noma K, et al. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med. 2014;24:165–9. - PubMed
-
- Cai H, Gehrig P, Scott TM, et al. MnSOD marks cord blood late outgrowth endothelial cells and accompanies robust resistance to oxidative stress. Biochem Biophys Res Commun. 2006;350:364–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

