In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy
- PMID: 25388867
- PMCID: PMC4229618
- DOI: 10.1186/s12968-014-0087-8
In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy
Abstract
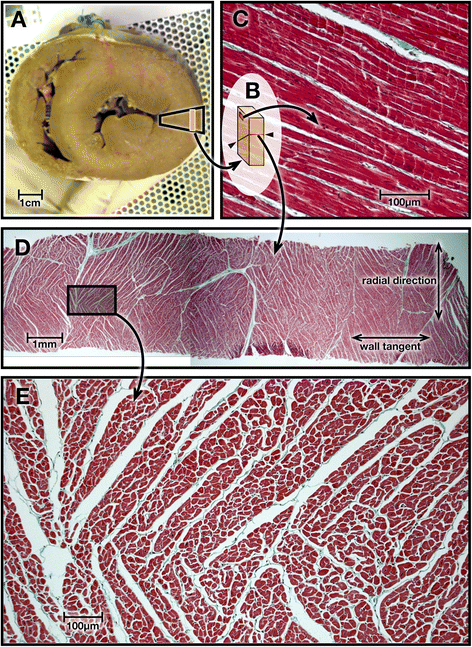
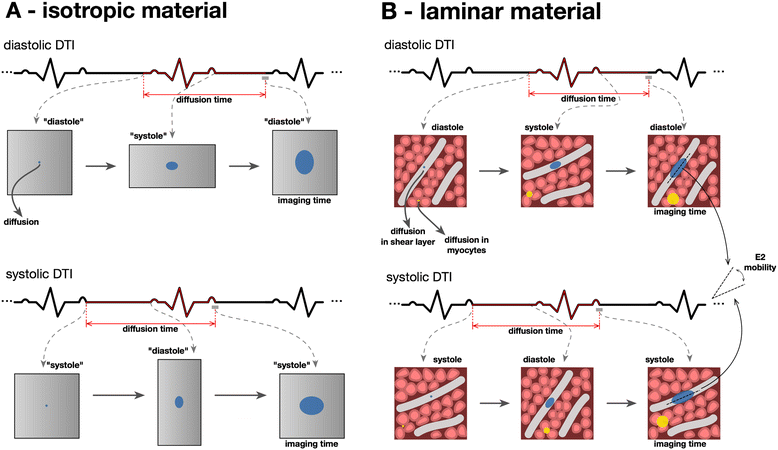
Background: Cardiac diffusion tensor imaging (cDTI) measures the magnitudes and directions of intramyocardial water diffusion. Assuming the cross-myocyte components to be constrained by the laminar microstructures of myocardium, we hypothesized that cDTI at two cardiac phases might identify any abnormalities of laminar orientation and mobility in hypertrophic cardiomyopathy (HCM).
Methods: We performed cDTI in vivo at 3 Tesla at end-systole and late diastole in 11 healthy controls and 11 patients with HCM, as well as late gadolinium enhancement (LGE) for detection of regional fibrosis.
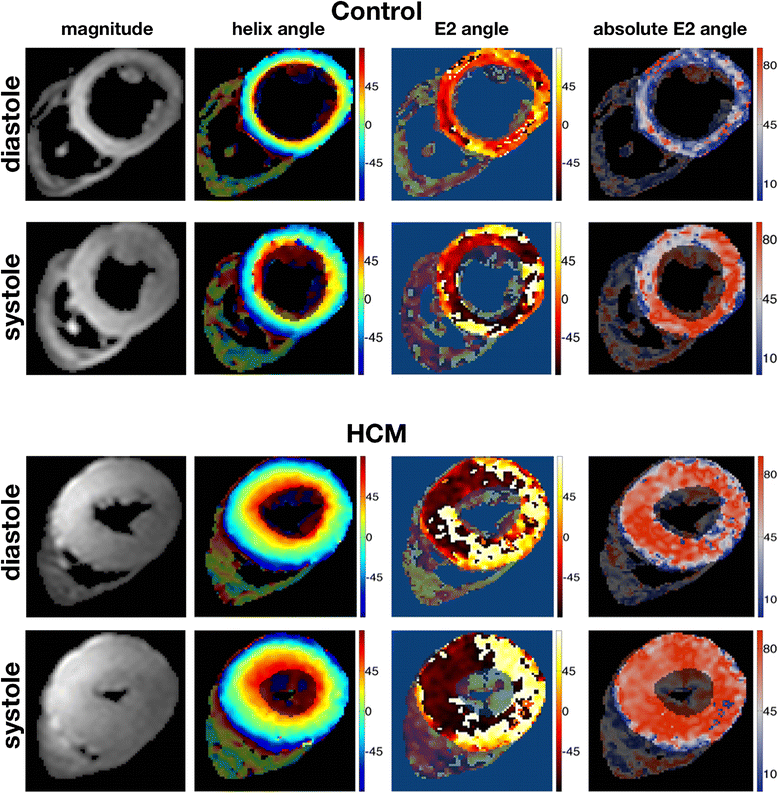
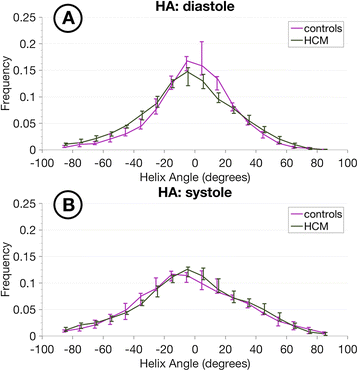
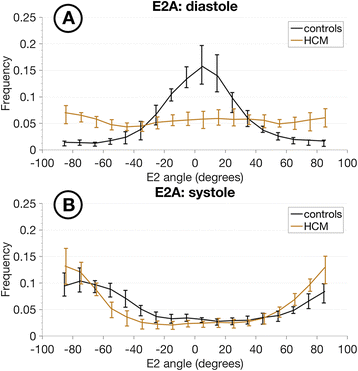
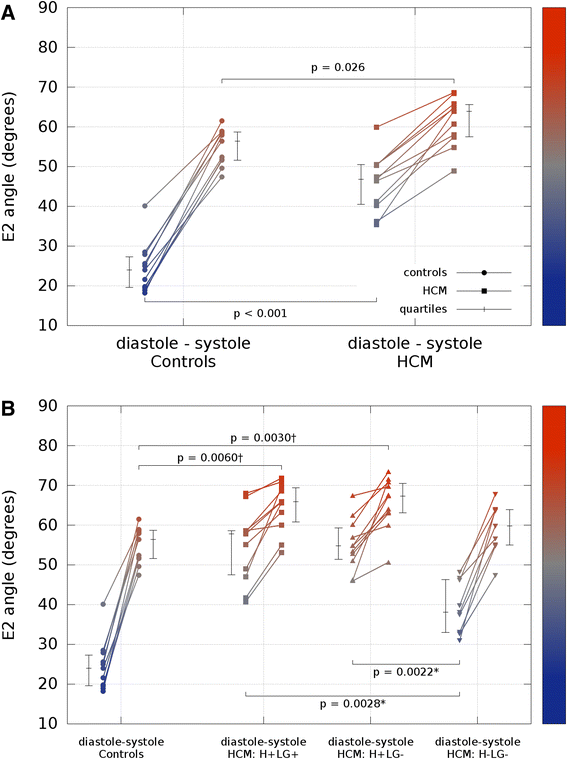
Results: Voxel-wise analysis of diffusion tensors relative to left ventricular coordinates showed expected transmural changes of myocardial helix-angle, with no significant differences between phases or between HCM and control groups. In controls, the angle of the second eigenvector of diffusion (E2A) relative to the local wall tangent plane was larger in systole than diastole, in accord with previously reported changes of laminar orientation. HCM hearts showed higher than normal global E2A in systole (63.9° vs 56.4° controls, p=0.026) and markedly raised E2A in diastole (46.8° vs 24.0° controls, p<0.001). In hypertrophic regions, E2A retained a high, systole-like angulation even in diastole, independent of LGE, while regions of normal wall thickness did not (LGE present 57.8°, p=0.0028, LGE absent 54.8°, p=0.0022 vs normal thickness 38.1°).
Conclusions: In healthy controls, the angles of cross-myocyte components of diffusion were consistent with previously reported transmural orientations of laminar microstructures and their changes with contraction. In HCM, especially in hypertrophic regions, they were consistent with hypercontraction in systole and failure of relaxation in diastole. Further investigation of this finding is required as previously postulated effects of strain might be a confounding factor.
Figures



Comment in
-
Probing dynamic myocardial microstructure with cardiac magnetic resonance diffusion tensor imaging.J Cardiovasc Magn Reson. 2014 Nov 12;16(1):89. doi: 10.1186/s12968-014-0089-6. J Cardiovasc Magn Reson. 2014. PMID: 25388937 Free PMC article.
References
-
- Pettigrew JB. On the arrangement of the muscular fibres in the ventricles of the vertebrate, with physiological remarks. Philos Trans. 1864;154:445–500. doi: 10.1098/rstl.1864.0014. - DOI
-
- Smerup M, Nielsen E, Agger P, Frandsen J, Vestergaard-Poulsen P, Andersen J, Nyengaard J, Pedersen M, Ringgaard S, Hjortdal V, Lunkenheimer PP, Anderson RH. The three-dimensional arrangement of the myocytes aggregated together within the mammalian ventricular myocardium. Anat Rec. 2009;292:1–11. doi: 10.1002/ar.20798. - DOI - PubMed
-
- Harrington KB, Rodriguez F, Cheng A, Langer F, Ashikaga H, Daughters GT, Criscione JC, Ingels NB, Miller DC. Direct measurement of transmural laminar architecture in the anterolateral wall of the ovine left ventricle: new implications for wall thickening mechanics. Am J Physiol Heart Circ Physiol. 2005;288:H1324–30. doi: 10.1152/ajpheart.00813.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

