Epidermal growth factor attenuates tubular necrosis following mercuric chloride damage by regeneration of indigenous, not bone marrow-derived cells
- PMID: 25389045
- PMCID: PMC4407604
- DOI: 10.1111/jcmm.12478
Epidermal growth factor attenuates tubular necrosis following mercuric chloride damage by regeneration of indigenous, not bone marrow-derived cells
Abstract
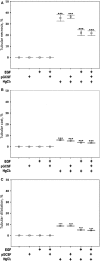
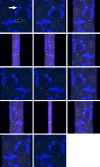
To assess effects of epidermal growth factor (EGF) and pegylated granulocyte colony-stimulating factor (P-GCSF; pegfilgrastim) administration on the cellular origin of renal tubular epithelium regenerating after acute kidney injury initiated by mercuric chloride (HgCl2 ). Female mice were irradiated and male whole bone marrow (BM) was transplanted into them. Six weeks later recipient mice were assigned to one of eight groups: control, P-GCSF+, EGF+, P-GCSF+EGF+, HgCl2 , HgCl2 +P-GCSF+, HgCl2 +EGF+ and HgCl2 +P-GCSF+EGF+. Following HgCl2 , injection tubular injury scores increased and serum urea nitrogen levels reached uraemia after 3 days, but EGF-treated groups were resistant to this acute kidney injury. A four-in-one analytical technique for identification of cellular origin, tubular phenotype, basement membrane and S-phase status revealed that BM contributed 1% of proximal tubular epithelium in undamaged kidneys and 3% after HgCl2 damage, with no effects of exogenous EGF or P-GCSF. Only 0.5% proximal tubular cells were seen in S-phase in the undamaged group kidneys; this increased to 7-8% after HgCl2 damage and to 15% after addition of EGF. Most of the regenerating tubular epithelium originated from the indigenous pool. BM contributed up to 6.6% of the proximal tubular cells in S-phase after HgCl2 damage, but only to 3.3% after additional EGF. EGF administration attenuated tubular necrosis following HgCl2 damage, and the major cause of this protective effect was division of indigenous cells, whereas BM-derived cells were less responsive. P-GCSF did not influence damage or regeneration.
Keywords: acute tubular necrosis; bone marrow-derived cells; epidermal growth factor; mercuric chloride; pegfilgrastim; tubular regeneration.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




References
-
- Norman J, Tsau YK, Bacay A, et al. Epidermal growth factor accelerates functional recovery from ischaemic acute tubular necrosis in the rat: role of the epidermal growth factor receptor. Clin Sci (Lond) 1990;78:445–50. - PubMed
-
- Coimbra TM, Cieslinski DA, Humes HD. Epidermal growth factor accelerates renal repair in mercuric chloride nephrotoxicity. Am J Physiol. 1990;259:F438–43. - PubMed
-
- Morin NJ, Laurent G, Nonclercq D, et al. Epidermal growth factor accelerates renal tissue repair in a model of gentamicin nephrotoxicity in rats. Am J Physiol. 1992;263:F806–11. - PubMed
-
- Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

