SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis
- PMID: 25389139
- PMCID: PMC4251206
- DOI: 10.1136/thoraxjnl-2014-205953
SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis
Abstract
Aim: Acute bronchiolitis is the commonest cause for hospitalisation in infancy. Supportive care remains the cornerstone of current management and no other therapy has been shown to influence the course of the disease. It has been suggested that adding nebulised hypertonic saline to usual care may shorten the duration of hospitalisation. To determine whether hypertonic saline does have beneficial effects we undertook an open, multi-centre parallel-group, pragmatic RCT in ten UK hospitals.
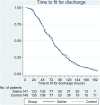
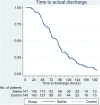
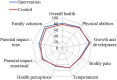
Methods: Infants admitted to hospital with a clinical diagnosis of acute bronchiolitis and requiring oxygen therapy were randomised to receive usual care alone or nebulised 3% hypertonic saline (HS) administered 6-hourly. Randomisation was within 4 h of admission. The primary outcome was time to being assessed as 'fit' for discharge with secondary outcomes including time to discharge, incidence of adverse events together with follow up to 28 days assessing patient centred health related outcomes.
Results: A total of 317 infants were recruited to the study. 158 infants were randomised to HS (141 analysed) and 159 to standard care (149 analysed). There was no difference between the two arms in time to being declared fit for discharge (hazard ratio: 0-95, 95% CI: 0.75-1.20) nor to actual discharge (hazard ratio: 0.97, 95% CI: 0.76-1.23). There was no difference in adverse events. One infant in the HS group developed bradycardia with desaturation.
Conclusion: This study does not support the use of nebulised HS in the treatment of acute bronchiolitis over usual care with minimal handlings.
Clinicaltrialsgov registration number: NCT01469845.
Keywords: Nebuliser therapy; Paediatric Lung Disaese; Viral infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Comment in
-
Nebulised hypertonic saline in bronchiolitis: take it with a pinch of salt.Thorax. 2014 Dec;69(12):1065-6. doi: 10.1136/thoraxjnl-2014-206210. Thorax. 2014. PMID: 25389137 No abstract available.
-
Nebulised hypertonic saline does not reduce hospital length of stay in acute bronchiolitis.Evid Based Med. 2015 Jun;20(3):106. doi: 10.1136/ebmed-2015-110168. Epub 2015 Mar 26. Evid Based Med. 2015. PMID: 25814124 No abstract available.
References
-
- Everard M. Respiratory syncytial virus bronchiolitis and pneumonia. In: Taussig L, Landau L, eds. Paediatric Respiratory Medicine. St Louis: Mosby, 2009:491–500.
-
- Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics 2010;125:342–9. - PubMed
-
- Martin AJ, Gardner PS, Mcquillin J. Epidemiology of respiratory viral infection among paediatric inpatients over a six-year period in North-East England. Lancet 1978;312:1035–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
