Synergistic modulation of cyclobutane pyrimidine dimer photoproduct formation and deamination at a TmCG site over a full helical DNA turn in a nucleosome core particle
- PMID: 25389265
- PMCID: PMC4245940
- DOI: 10.1093/nar/gku1049
Synergistic modulation of cyclobutane pyrimidine dimer photoproduct formation and deamination at a TmCG site over a full helical DNA turn in a nucleosome core particle
Abstract
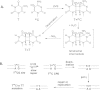
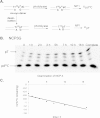
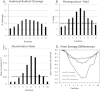
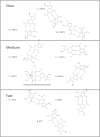
Sunlight-induced C to T mutation hotspots in skin cancers occur primarily at methylated CpG sites that coincide with sites of UV-induced cyclobutane pyrimidine dimer (CPD) formation. The C or 5-methyl-C in CPDs are not stable and deaminate to U and T, respectively, which leads to the insertion of A by DNA polymerase η and defines a probable mechanism for the origin of UV-induced C to T mutations. We have now determined the photoproduct formation and deamination rates for 10 consecutive T=(m)CG CPDs over a full helical turn at the dyad axis of a nucleosome and find that whereas photoproduct formation and deamination is greatly inhibited for the CPDs closest to the histone surface, it is greatly enhanced for the outermost CPDs. Replacing the G in a T=(m)CG CPD with A greatly decreased the deamination rate. These results show that rotational position and flanking sequence in a nucleosome can significantly and synergistically modulate CPD formation and deamination that contribute to C to T mutations associated with skin cancer induction and may have influenced the evolution of the human genome.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Rapid deamination of cyclobutane pyrimidine dimer photoproducts at TCG sites in a translationally and rotationally positioned nucleosome in vivo.J Biol Chem. 2015 Oct 30;290(44):26597-609. doi: 10.1074/jbc.M115.673301. Epub 2015 Sep 9. J Biol Chem. 2015. PMID: 26354431 Free PMC article.
-
Rotational position of a 5-methylcytosine-containing cyclobutane pyrimidine dimer in a nucleosome greatly affects its deamination rate.J Biol Chem. 2011 Feb 25;286(8):6329-35. doi: 10.1074/jbc.M110.183178. Epub 2010 Dec 15. J Biol Chem. 2011. PMID: 21160086 Free PMC article.
-
Modulation of cyclobutane thymine photodimer formation in T11-tracts in rotationally phased nucleosome core particles and DNA minicircles.Nucleic Acids Res. 2017 Jul 7;45(12):7031-7041. doi: 10.1093/nar/gkx427. Nucleic Acids Res. 2017. PMID: 28525579 Free PMC article.
-
Solar UV radiation-induced DNA Bipyrimidine photoproducts: formation and mechanistic insights.Top Curr Chem. 2015;356:249-75. doi: 10.1007/128_2014_553. Top Curr Chem. 2015. PMID: 25370518 Review.
-
Damage mapping techniques and the light they have shed on canonical and atypical UV photoproducts.Front Genet. 2023 Jan 10;13:1102593. doi: 10.3389/fgene.2022.1102593. eCollection 2022. Front Genet. 2023. PMID: 36704334 Free PMC article. Review.
Cited by
-
Histone Tail Sequences Balance Their Role in Genetic Regulation and the Need To Protect DNA against Destruction in Nucleosome Core Particles Containing Abasic Sites.Chembiochem. 2019 Jan 2;20(1):78-82. doi: 10.1002/cbic.201800559. Epub 2018 Nov 15. Chembiochem. 2019. PMID: 30307690 Free PMC article.
-
Organization of DNA damage, excision repair, and mutagenesis in chromatin: A genomic perspective.DNA Repair (Amst). 2019 Sep;81:102645. doi: 10.1016/j.dnarep.2019.102645. Epub 2019 Jul 8. DNA Repair (Amst). 2019. PMID: 31307926 Free PMC article. Review.
-
5-Formylcytosine Yields DNA-Protein Cross-Links in Nucleosome Core Particles.J Am Chem Soc. 2017 Aug 9;139(31):10617-10620. doi: 10.1021/jacs.7b05495. Epub 2017 Jul 25. J Am Chem Soc. 2017. PMID: 28742335 Free PMC article.
-
Oxidation of 8-Oxo-7,8-dihydro-2'-deoxyguanosine Leads to Substantial DNA-Histone Cross-Links within Nucleosome Core Particles.Chem Res Toxicol. 2018 Dec 17;31(12):1364-1372. doi: 10.1021/acs.chemrestox.8b00244. Epub 2018 Nov 19. Chem Res Toxicol. 2018. PMID: 30412392 Free PMC article.
-
Frequency and Spectrum of Mutations Induced by Gamma Rays Revealed by Phenotype Screening and Whole-Genome Re-Sequencing in Arabidopsis thaliana.Int J Mol Sci. 2022 Jan 7;23(2):654. doi: 10.3390/ijms23020654. Int J Mol Sci. 2022. PMID: 35054839 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

