Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin
- PMID: 25389296
- PMCID: PMC4281761
- DOI: 10.1074/jbc.M114.616904
Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin
Abstract
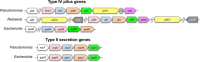
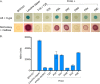
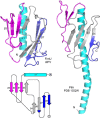
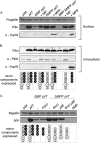
Type IV pili (T4P) contain hundreds of major subunits, but minor subunits are also required for assembly and function. Here we show that Pseudomonas aeruginosa minor pilins prime pilus assembly and traffic the pilus-associated adhesin and anti-retraction protein, PilY1, to the cell surface. PilV, PilW, and PilX require PilY1 for inclusion in surface pili and vice versa, suggestive of complex formation. PilE requires PilVWXY1 for inclusion, suggesting that it binds a novel interface created by two or more components. FimU is incorporated independently of the others and is proposed to couple the putative minor pilin-PilY1 complex to the major subunit. The production of small amounts of T4P by a mutant lacking the minor pilin operon was traced to expression of minor pseudopilins from the P. aeruginosa type II secretion (T2S) system, showing that under retraction-deficient conditions, T2S minor subunits can prime T4P assembly. Deletion of all minor subunits abrogated pilus assembly. In a strain lacking the minor pseudopilins, PilVWXY1 and either FimU or PilE comprised the minimal set of components required for pilus assembly. Supporting functional conservation of T2S and T4P minor components, our 1.4 Å crystal structure of FimU revealed striking architectural similarity to its T2S ortholog GspH, despite minimal sequence identity. We propose that PilVWXY1 form a priming complex for assembly and that PilE and FimU together stably couple the complex to the major subunit. Trafficking of the anti-retraction factor PilY1 to the cell surface allows for production of pili of sufficient length to support adherence and motility.
Keywords: Adhesin; Bacterial Adhesion; Crystal Structure; Pilin; Pseudomonas aeruginosa (P. aeruginosa); Pseudopilin; Twitching Motility; Type II Secretion System (T2SS); Type IV Pili.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Pelicic V. (2008) Type IV pili: e pluribus unum? Mol. Microbiol. 68, 827–837 - PubMed
-
- Burrows L. L. (2012) Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66, 493–520 - PubMed
-
- Craig L., Pique M. E., Tainer J. A. (2004) Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2, 363–378 - PubMed
-
- Mattick J. S. (2002) Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

