Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback
- PMID: 25389309
- PMCID: PMC4250167
- DOI: 10.1073/pnas.1419045111
Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback
Abstract
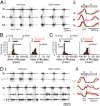
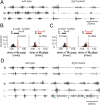
Mammalian locomotor programs are thought to be directed by the actions of spinal interneuron circuits collectively referred to as "central pattern generators." The contribution of proprioceptive sensory feedback to the coordination of locomotor activity remains less clear. We have analyzed changes in mouse locomotor pattern under conditions in which proprioceptive feedback is attenuated genetically and biomechanically. We find that locomotor pattern degrades upon elimination of proprioceptive feedback from muscle spindles and Golgi tendon organs. The degradation of locomotor pattern is manifest as the loss of interjoint coordination and alternation of flexor and extensor muscles. Group Ia/II sensory feedback from muscle spindles has a predominant influence in patterning the activity of flexor muscles, whereas the redundant activities of group Ia/II and group Ib afferents appear to determine the pattern of extensor muscle firing. These findings establish a role for proprioceptive feedback in the control of fundamental aspects of mammalian locomotor behavior.
Keywords: locomotion; pattern generation; proprioception; sensory feedback.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks V, editor. Handbook of Physiology: The Nervous System, Motor Control. Am Physiol Soc; Bethesda, MD: 1981. Vol 2, pp 1176–1236.
-
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969;75(4):614–630. - PubMed
-
- Prochazka A, Trend P, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: Towards a representative look-up chart. Prog Brain Res. 1989;80:61–74, discussion 57–60. - PubMed
-
- Rossignol S. 1996. Neural control of stereotypic limb movements. In Handbook of Physiology, Section 12. Exercise: Regulation and Integration of Multiple Systems, eds Rowell LB and Shepherd JT (Am Physiol Soc, Bethesda, MD), pp 173–216.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

