Enhancement of sleep slow waves: underlying mechanisms and practical consequences
- PMID: 25389394
- PMCID: PMC4211398
- DOI: 10.3389/fnsys.2014.00208
Enhancement of sleep slow waves: underlying mechanisms and practical consequences
Abstract
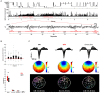
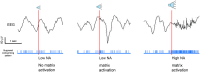
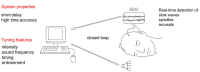
Even modest sleep restriction, especially the loss of sleep slow wave activity (SWA), is invariably associated with slower electroencephalogram (EEG) activity during wake, the occurrence of local sleep in an otherwise awake brain, and impaired performance due to cognitive and memory deficits. Recent studies not only confirm the beneficial role of sleep in memory consolidation, but also point to a specific role for sleep slow waves. Thus, the implementation of methods to enhance sleep slow waves without unwanted arousals or lightening of sleep could have significant practical implications. Here we first review the evidence that it is possible to enhance sleep slow waves in humans using transcranial direct-current stimulation (tDCS) and transcranial magnetic stimulation. Since these methods are currently impractical and their safety is questionable, especially for chronic long-term exposure, we then discuss novel data suggesting that it is possible to enhance slow waves using sensory stimuli. We consider the physiology of the K-complex (KC), a peripheral evoked slow wave, and show that, among different sensory modalities, acoustic stimulation is the most effective in increasing the magnitude of slow waves, likely through the activation of non-lemniscal ascending pathways to the thalamo-cortical system. In addition, we discuss how intensity and frequency of the acoustic stimuli, as well as exact timing and pattern of stimulation, affect sleep enhancement. Finally, we discuss automated algorithms that read the EEG and, in real-time, adjust the stimulation parameters in a closed-loop manner to obtain an increase in sleep slow waves and avoid undesirable arousals. In conclusion, while discussing the mechanisms that underlie the generation of sleep slow waves, we review the converging evidence showing that acoustic stimulation is safe and represents an ideal tool for slow wave sleep (SWS) enhancement.
Keywords: EEG; NREM sleep; acoustic stimulation; arousal systems; closed-loop.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

