Long-term virological outcome in children on antiretroviral therapy in the UK and Ireland
- PMID: 25389551
- PMCID: PMC4210689
- DOI: 10.1097/qad.0000000000000438
Long-term virological outcome in children on antiretroviral therapy in the UK and Ireland
Abstract
Objective: To assess factors at the start of antiretroviral therapy (ART) associated with long-term virological response in children.
Design: Multicentre national cohort.
Methods: Factors associated with viral load below 400 copies/ml by 12 months and virologic failure among children starting 3/4-drug ART in the UK/Irish Collaborative HIV Paediatric Study were assessed using Poisson models.
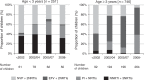
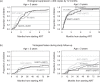
Results: Nine hundred and ninety-seven children started ART at a median age of 7.7 years (inter-quartile range 2.9–11.7), 251 (25%) below 3 years: 411 (41%) with efavirenz and two nucleoside reverse transcriptase inhibitors (EFVþ2NRTIs), 264 (26%) with nevirapine and two NRTIs (NVPþ2NRTIs), 119 (12%; 106 NVP, 13 EFV) with non-nucleoside reverse transcriptase inhibitor and three NRTIs (NNRTIþ3NRTIs), and 203 (20%) with boosted protease inhibitor-based regimens. Median follow-up after ART initiation was 5.7 (3.0–8.8) years. Viral load was less than 400 copies/ml by 12 months in 92% [95% confidence interval (CI) 91–94%] of the children. Time to suppression was similar across regimens (P¼0.10), but faster over calendar time, with older age and lower baseline viral load. Three hundred and thirtynine (34%) children experienced virological failure. Although progression to failure varied by regimen (P<0.001) and was fastest for NVPþ2NRTIs regimens, risk after 2 years on therapy was similar for EFVþ2NRTIs and NVPþ2NRTIs, and lowest for NNRTIþ3NRTIs regimens (P-interaction¼0.03). Older age, earlier calendar periods and maternal ART exposure were associated with increased failure risk. Early treatment discontinuation for toxicity occurred more frequently for NVP-based regimens, but 5-year cumulative incidence was similar: 6.1% (95% CI 3.9–8.9%) NVP, 8.3% (95% CI 5.6–11.6) EFV, and 9.8% (95% CI 5.7–15.3%) protease inhibitor-based regimens (P¼0.48).
Conclusion: Viral load suppression by 12 months was high with all regimens. NVPþ3NRTIs regimens were particularly efficacious in the longer term and may be a good alternative to protease inhibitor-based ART in young children.
2014 Wolters Kluwer Health | Lippincott Williams & Wilkins
Figures
References
-
- Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis 2011; 11:769–779 - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. June 2013. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [Accessed August 2013] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical